Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial
- PMID: 27375040
- PMCID: PMC5351775
- DOI: 10.1016/S0140-6736(16)30371-3
Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial
Abstract
Background: Safety and efficacy have been shown in a phase 1 dose-escalation study involving a unilateral subretinal injection of a recombinant adeno-associated virus (AAV) vector containing the RPE65 gene (AAV2-hRPE65v2) in individuals with inherited retinal dystrophy caused by RPE65 mutations. This finding, along with the bilateral nature of the disease and intended use in treatment, prompted us to determine the safety of administration of AAV2-hRPE65v2 to the contralateral eye in patients enrolled in the phase 1 study.
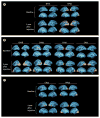
Methods: In this follow-on phase 1 trial, one dose of AAV2-hRPE65v2 (1.5 × 10(11) vector genomes) in a total volume of 300 μL was subretinally injected into the contralateral, previously uninjected, eyes of 11 children and adults (aged 11-46 years at second administration) with inherited retinal dystrophy caused by RPE65 mutations, 1.71-4.58 years after the initial subretinal injection. We assessed safety, immune response, retinal and visual function, functional vision, and activation of the visual cortex from baseline until 3 year follow-up, with observations ongoing. This study is registered with ClinicalTrials.gov, number NCT01208389.
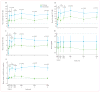
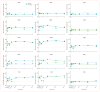
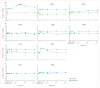
Findings: No adverse events related to the AAV were reported, and those related to the procedure were mostly mild (dellen formation in three patients and cataracts in two). One patient developed bacterial endophthalmitis and was excluded from analyses. We noted improvements in efficacy outcomes in most patients without significant immunogenicity. Compared with baseline, pooled analysis of ten participants showed improvements in mean mobility and full-field light sensitivity in the injected eye by day 30 that persisted to year 3 (mobility p=0.0003, white light full-field sensitivity p<0.0001), but no significant change was seen in the previously injected eyes over the same time period (mobility p=0.7398, white light full-field sensitivity p=0.6709). Changes in visual acuity from baseline to year 3 were not significant in pooled analysis in the second eyes or the previously injected eyes (p>0.49 for all time-points compared with baseline).
Interpretation: To our knowledge, AAV2-hRPE65v2 is the first successful gene therapy administered to the contralateral eye. The results highlight the use of several outcome measures and help to delineate the variables that contribute to maximal benefit from gene augmentation therapy in this disease.
Funding: Center for Cellular and Molecular Therapeutics at The Children's Hospital of Philadelphia, Spark Therapeutics, US National Institutes of Health, Foundation Fighting Blindness, Institute for Translational Medicine and Therapeutics, Research to Prevent Blindness, Center for Advanced Retinal and Ocular Therapeutics, Mackall Foundation Trust, F M Kirby Foundation, and The Research Foundation-Flanders.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
AMM and JB are co-inventors on a patent for “a method of treating or retarding the development of blindness” (US Patent number 8147823) but waived any potential financial interest in this technology in 2002. KAH, JFW, DCC, and JW are now employed by and have equity in Spark Therapeutics, a company that was formed after the participants had received intervention to the second eye and that is developing this technology. JB also reports having served on a scientific advisory board for Avalanche Technologies and is a founder of Gensight Biologics. JB, DCC, AMM, KAH, JW, KAM, SM, and JS are coauthors of a provisional patent describing the mobility test used in this study. FM and KAH hold a patent on methods for detection and modulation of T-cell responses to gene therapy vectors. JFW is an inventor on patents relative to adeno-associated vector development. All other authors declare no competing interests.
Figures



Comment in
-
Benefits of gene therapy for both eyes.Lancet. 2016 Aug 13;388(10045):635-6. doi: 10.1016/S0140-6736(16)30783-8. Epub 2016 Jun 30. Lancet. 2016. PMID: 27375039 No abstract available.
-
Vision quest: gene therapy for inherited vision loss.Lancet. 2018 Jan 6;391(10115):2. doi: 10.1016/S0140-6736(18)30004-7. Lancet. 2018. PMID: 29323644 No abstract available.
Similar articles
-
Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial.Lancet. 2009 Nov 7;374(9701):1597-605. doi: 10.1016/S0140-6736(09)61836-5. Epub 2009 Oct 23. Lancet. 2009. PMID: 19854499 Free PMC article. Clinical Trial.
-
Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial.Lancet. 2017 Aug 26;390(10097):849-860. doi: 10.1016/S0140-6736(17)31868-8. Epub 2017 Jul 14. Lancet. 2017. PMID: 28712537 Free PMC article. Clinical Trial.
-
Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation-Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3 Trials.Ophthalmology. 2019 Sep;126(9):1273-1285. doi: 10.1016/j.ophtha.2019.06.017. Epub 2019 Jun 22. Ophthalmology. 2019. PMID: 31443789 Clinical Trial.
-
Voretigene Neparvovec: A Review in RPE65 Mutation-Associated Inherited Retinal Dystrophy.Mol Diagn Ther. 2020 Aug;24(4):487-495. doi: 10.1007/s40291-020-00475-6. Mol Diagn Ther. 2020. PMID: 32535767 Review.
-
The Status of RPE65 Gene Therapy Trials: Safety and Efficacy.Cold Spring Harb Perspect Med. 2015 Jan 29;5(9):a017285. doi: 10.1101/cshperspect.a017285. Cold Spring Harb Perspect Med. 2015. PMID: 25635059 Free PMC article. Review.
Cited by
-
Delivery Approaches for Therapeutic Genome Editing and Challenges.Genes (Basel). 2020 Sep 23;11(10):1113. doi: 10.3390/genes11101113. Genes (Basel). 2020. PMID: 32977396 Free PMC article. Review.
-
Splice-Modulating Oligonucleotide QR-110 Restores CEP290 mRNA and Function in Human c.2991+1655A>G LCA10 Models.Mol Ther Nucleic Acids. 2018 Sep 7;12:730-740. doi: 10.1016/j.omtn.2018.07.010. Epub 2018 Jul 23. Mol Ther Nucleic Acids. 2018. PMID: 30114557 Free PMC article.
-
Gene manipulation in liver ductal organoids by optimized recombinant adeno-associated virus vectors.J Biol Chem. 2019 Sep 20;294(38):14096-14104. doi: 10.1074/jbc.RA119.008616. Epub 2019 Jul 31. J Biol Chem. 2019. PMID: 31366731 Free PMC article.
-
The Effect of CpG Sequences on Capsid-Specific CD8+ T Cell Responses to AAV Vector Gene Transfer.Mol Ther. 2020 Mar 4;28(3):771-783. doi: 10.1016/j.ymthe.2019.11.014. Epub 2019 Nov 21. Mol Ther. 2020. PMID: 31839483 Free PMC article.
-
The Degree of Adeno-Associated Virus-Induced Retinal Inflammation Varies Based on Serotype and Route of Delivery: Intravitreal, Subretinal, or Suprachoroidal.Hum Gene Ther. 2023 Jun;34(11-12):530-539. doi: 10.1089/hum.2022.222. Epub 2023 Mar 28. Hum Gene Ther. 2023. PMID: 36793189 Free PMC article.
References
-
- Thompson DA, Gyurus P, Fleischer LL, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2000;41:4293–99. - PubMed
-
- Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–51. - PubMed
-
- Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

