The activation of OR51E1 causes growth suppression of human prostate cancer cells
- PMID: 27374083
- PMCID: PMC5217014
- DOI: 10.18632/oncotarget.10197
The activation of OR51E1 causes growth suppression of human prostate cancer cells
Abstract
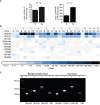
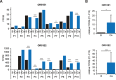
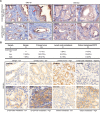
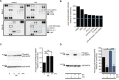
The development of prostate cancer (PCa) is regulated by the androgen-dependent activity of the androgen receptor (AR). Androgen-deprivation therapy (ADT) is therefore the gold standard treatment to suppress malignant progression of PCa. Nevertheless, due to the development of castration resistance, recurrence of disease after initial response to ADT is a major obstacle to successful treatment. As G-protein coupled receptors play a fundamental role in PCa physiology, they might represent promising alternative or combinatorial targets for advanced diseases. Here, we verified gene expression of the olfactory receptors (ORs) OR51E1 [prostate-specific G-protein coupled receptor 2 (PSGR2)] and OR51E2 (PSGR) in human PCa tissue by RNA-Seq analysis and RT-PCR and elucidated the subcellular localization of both receptor proteins in human prostate tissue. The OR51E1 agonist nonanoic acid (NA) leads to the phosphorylation of various protein kinases and growth suppression of the PCa cell line LNCaP. Furthermore, treatment with NA causes reduction of androgen-mediated AR target gene expression. Interestingly, NA induces cellular senescence, which coincides with reduced E2F1 mRNA levels. In contrast, treatment with the structurally related compound 1-nonanol or the OR2AG1 agonist amyl butyrate, neither of which activates OR51E1, did not lead to reduced cell growth or an induction of cellular senescence. However, decanoic acid, another OR51E1 agonist, also induces cellular senescence. Thus, our results suggest the involvement of OR51E1 in growth processes of PCa cells and its impact on AR-mediated signaling. These findings provide novel evidences to support the functional importance of ORs in PCa pathogenesis.
Keywords: OR51E1; androgen receptor; cellular senescence; proliferation; prostate cancer.
Conflict of interest statement
The authors declare that there is no conflicts of interest.
Figures

Similar articles
-
Ectopically expressed olfactory receptors OR51E1 and OR51E2 suppress proliferation and promote cell death in a prostate cancer cell line.J Biol Chem. 2021 Jan-Jun;296:100475. doi: 10.1016/j.jbc.2021.100475. Epub 2021 Feb 26. J Biol Chem. 2021. PMID: 33640452 Free PMC article.
-
The androgen receptor-lncRNASAT1-AKT-p15 axis mediates androgen-induced cellular senescence in prostate cancer cells.Oncogene. 2022 Feb;41(7):943-959. doi: 10.1038/s41388-021-02060-5. Epub 2021 Oct 19. Oncogene. 2022. PMID: 34667276 Free PMC article.
-
The tumor suppressor ING1b is a novel corepressor for the androgen receptor and induces cellular senescence in prostate cancer cells.J Mol Cell Biol. 2016 Jun;8(3):207-20. doi: 10.1093/jmcb/mjw007. Epub 2016 Mar 18. J Mol Cell Biol. 2016. PMID: 26993046
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Regulation of tumor cell plasticity by the androgen receptor in prostate cancer.Endocr Relat Cancer. 2015 Jun;22(3):R165-82. doi: 10.1530/ERC-15-0137. Epub 2015 May 1. Endocr Relat Cancer. 2015. PMID: 25934687 Review.
Cited by
-
Ectopically expressed olfactory receptors OR51E1 and OR51E2 suppress proliferation and promote cell death in a prostate cancer cell line.J Biol Chem. 2021 Jan-Jun;296:100475. doi: 10.1016/j.jbc.2021.100475. Epub 2021 Feb 26. J Biol Chem. 2021. PMID: 33640452 Free PMC article.
-
Odorant receptors in cancer.BMB Rep. 2022 Feb;55(2):72-80. doi: 10.5483/BMBRep.2022.55.2.010. BMB Rep. 2022. PMID: 35168702 Free PMC article. Review.
-
Exposure to nonanoic acid alters small intestinal neuroendocrine tumor phenotype.BMC Cancer. 2023 Mar 23;23(1):267. doi: 10.1186/s12885-023-10722-8. BMC Cancer. 2023. PMID: 36959559 Free PMC article.
-
Olfactory Receptors as an Emerging Chemical Sensing Scaffold.Biochemistry. 2023 Jan 17;62(2):187-195. doi: 10.1021/acs.biochem.2c00486. Epub 2022 Nov 1. Biochemistry. 2023. PMID: 36318941 Free PMC article. Review.
-
Revisiting olfactory receptors as putative drivers of cancer.Wellcome Open Res. 2017 Feb 10;2:9. doi: 10.12688/wellcomeopenres.10646.1. Wellcome Open Res. 2017. PMID: 28492065 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

