Aminosalicylates for induction of remission or response in Crohn's disease
- PMID: 27372735
- PMCID: PMC6457996
- DOI: 10.1002/14651858.CD008870.pub2
Aminosalicylates for induction of remission or response in Crohn's disease
Abstract
Background: Randomized trials investigating the efficacy of aminosalicylates for the treatment of mildly to moderately active Crohn's disease have yielded conflicting results. A systematic review was conducted to critically examine current available data on the efficacy of sulfasalazine and mesalamine for inducing remission or clinical response in these patients.
Objectives: To evaluate the efficacy of aminosalicylates compared to placebo, corticosteroids, and other aminosalicylates (alone or in combination with corticosteroids) for the treatment of mildly to moderately active Crohn's disease.
Search methods: We searched PubMed, EMBASE, MEDLINE and the Cochrane Central Library from inception to June 2015 to identify relevant studies. There were no language restrictions. We also searched reference lists from potentially relevant papers and review articles, as well as proceedings from annual meetings (1991-2015) of the American Gastroenterological Association and American College of Gastroenterology.
Selection criteria: Randomized controlled trials that evaluated the efficacy of sulfasalazine or mesalamine in the treatment of mildly to moderately active Crohn's disease compared to placebo, corticosteroids, and other aminosalicylates (alone or in combination with corticosteroids) were included.
Data collection and analysis: Data extraction and assessment of methodological quality was independently performed by the investigators and any disagreement was resolved by discussion and consensus. We assessed methodological quality using the Cochrane risk of bias tool. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria. The primary outcome measure was a well defined clinical endpoint of induction of remission or response to treatment. Secondary outcomes included mean Crohn's disease activity index (CDAI) scores, adverse events, serious adverse events and withdrawal due to adverse events. For dichotomous outcomes we calculated the pooled risk ratio (RR) and corresponding 95% confidence interval (CI) using a random-effects model. For continuous outcomes we calculated the mean difference (MD) and 95% CI using a random-effects model. Sensitivity analyses based on a fixed-effect model and duration of therapy were conducted where appropriate.
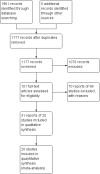
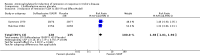
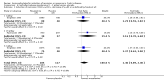
Main results: Twenty studies (2367 patients) were included. Two studies were judged to be at high risk of bias due to lack of blinding. Eight studies were judged to be at high risk of bias due to incomplete outcomes data (high drop-out rates) and potential selective reporting. The other 10 studies were judged to be at low risk of bias. A non-significant trend in favour of sulfasalazine over placebo for inducing remission was observed, with benefit confined mainly to patients with Crohn's colitis. Forty-five per cent (63/141) of sulfasalazine patients entered remission at 17-18 weeks compared to 29% (43/148) of placebo patients (RR 1.38, 95% CI 1.00 to 1.89, 2 studies). A GRADE analysis rated the overall quality of the evidence supporting this outcome as moderate due to sparse data (106 events). There was no difference between sulfasalazine and placebo in adverse event outcomes. Sulfasalazine was significantly less effective than corticosteroids and inferior to combination therapy with corticosteroids (RR 0.64, 95% CI 0.47 to 0.86, 1 study, 110 patients). Forty-three per cent (55/128) of sulfasalazine patients entered remission at 17 to 18 weeks compared to 60% (79/132) of corticosteroid patients (RR 0.68, 95% CI 0.51 to 0.91; 2 studies, 260 patients). A GRADE analysis rated the overall quality of the evidence supporting this outcome as moderate due to sparse data (134 events). Sulfasalazine patients experienced significantly fewer adverse events than corticosteroid patients (RR 0.43, 95% CI 0.22 to 0.82; 1 study, 159 patients). There was no difference between sulfasalazine and corticosteroids in serious adverse events or withdrawal due to adverse events. Olsalazine was less effective than placebo in a single trial (RR 0.36, 95% CI 0.18 to 0.71; 91 patients). Low dose mesalamine (1 to 2 g/day) was not superior to placebo for induction of remission. Twenty-three per cent (43/185) of low dose mesalamine patients entered remission at week 6 compared to 15% (18/117) of placebo patients (RR = 1.46, 95% CI 0.89 to 2.40; n = 302). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to risk of bias (incomplete outcome data) and sparse data (61 events). There was no difference between low dose mesalamine and placebo in the proportion of patients who had adverse events (RR 1.33, 95% CI 0.91 to 1.96; 3 studies, 342 patients) or withdrew due to adverse events (RR 1.21, 95% CI 0.75 to 1.95; 3 studies, 342 patients). High dose controlled-release mesalamine (4 g/day) was not superior to placebo, inducing a clinically non significant reduction in CDAI (MD -19.8 points, 95% CI -46.2 to 6.7; 3 studies, 615 patients), and was also inferior to budesonide (RR 0.56, 95% CI 0.40 to 0.78; 1 study, 182 patients, GRADE = low). While high dose delayed-release mesalamine (3 to 4.5 g/day) was not superior to placebo for induction of remission (RR 2.02, 95% CI 0.75 to 5.45; 1 study, 38 patients, GRADE = very low), no significant difference in efficacy was found when compared to conventional corticosteroids (RR 1.04, 95% CI 0.79 to 1.36; 3 studies, 178 patients, GRADE = moderate) or budesonide (RR 0.89, 95% CI 0.76 to 1.05; 1 study, 307 patients, GRADE = moderate). However, these trials were limited by risk of bias (incomplete outcome data) and sparse data (small numbers of events). There was a lack of good quality clinical trials comparing sulfasalazine with other mesalamine formulations. Adverse events that were commonly reported included headache, nausea, vomiting, abdominal pain and diarrhea.
Authors' conclusions: Sulfasalazine is only modestly effective with a trend towards benefit over placebo and is inferior to corticosteroids for the treatment of mildly to moderately active Crohn's disease. Olsalazine and low dose mesalamine (1 to 2 g/day) are not superior to placebo. High dose mesalamine (3.2 to 4 g/day) is not more effective than placebo for inducing response or remission. However, trials assessing the efficacy of high dose mesalamine (4 to 4.5 g/day) compared to budesonide yielded conflicting results and firm conclusions cannot be made. Future large randomized controlled trials are needed to provide definitive evidence on the efficacy of aminosalicylates in active Crohn's disease.
Conflict of interest statement
Wee‐Chian Lim: Wee‐Chian Lim's institution has received funds from Takeda Pharmaceuticals (Asia Pacific) Pte. Ltd, JANSSEN ASIA PACIFIC, a division of Johnson & Johnson Pte Ltd, and Abbvie Pte Ltd for participant in Advisory Boards. All of these financial activities are outside of the scope of the present review.
Yongjun Wang: None known
John MacDonald: None known
Stephen Hanauer: Stephen Hanauer has received funds from Abbvie, Cellgene, Janssen, Ferring, Takeda, Pfizer, Merk, UCB, Actavis, and Shire for consultancy; and fees from Abbvie, Takeda and Janssen as payment for lectures; and travel expenses from Falk Foundation. All of these financial activities are outside of the scope of the present review.
Figures


































Update of
-
Aminosalicylates for induction of remission or response in Crohn's disease.Cochrane Database Syst Rev. 2010 Dec 8;(12):CD008870. doi: 10.1002/14651858.CD008870. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2016 Jul 03;7:CD008870. doi: 10.1002/14651858.CD008870.pub2. PMID: 21154400 Updated. Review.
Similar articles
-
Aminosalicylates for induction of remission or response in Crohn's disease.Cochrane Database Syst Rev. 2010 Dec 8;(12):CD008870. doi: 10.1002/14651858.CD008870. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2016 Jul 03;7:CD008870. doi: 10.1002/14651858.CD008870.pub2. PMID: 21154400 Updated. Review.
-
Budesonide for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2015 Jun 3;2015(6):CD000296. doi: 10.1002/14651858.CD000296.pub4. Cochrane Database Syst Rev. 2015. PMID: 26039678 Free PMC article. Review.
-
Budesonide for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2014 Aug 21;2014(8):CD002913. doi: 10.1002/14651858.CD002913.pub3. Cochrane Database Syst Rev. 2014. PMID: 25141071 Free PMC article. Review.
-
Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2016 Oct 26;10(10):CD000545. doi: 10.1002/14651858.CD000545.pub5. Cochrane Database Syst Rev. 2016. PMID: 27783843 Free PMC article. Review.
-
Methotrexate for induction of remission in refractory Crohn's disease.Cochrane Database Syst Rev. 2014 Aug 6;2014(8):CD003459. doi: 10.1002/14651858.CD003459.pub4. Cochrane Database Syst Rev. 2014. PMID: 25099640 Free PMC article. Review.
Cited by
-
Natural history of children with mild Crohn's disease.World J Gastroenterol. 2019 Aug 14;25(30):4235-4245. doi: 10.3748/wjg.v25.i30.4235. World J Gastroenterol. 2019. PMID: 31435176 Free PMC article.
-
Systematic review: medical therapy for fibrostenosing Crohn's disease.Aliment Pharmacol Ther. 2020 Jun;51(12):1233-1246. doi: 10.1111/apt.15750. Epub 2020 May 13. Aliment Pharmacol Ther. 2020. PMID: 32406116 Free PMC article.
-
A review of the therapeutic management of Crohn's disease.Therap Adv Gastroenterol. 2022 Feb 17;15:17562848221078456. doi: 10.1177/17562848221078456. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 35198041 Free PMC article. Review.
-
Regulation of the Hypoxia-Inducible Factor (HIF) by Pro-Inflammatory Cytokines.Cells. 2021 Sep 7;10(9):2340. doi: 10.3390/cells10092340. Cells. 2021. PMID: 34571989 Free PMC article. Review.
-
5-Aminosalicylates to maintain remission in Crohn's disease: Interpreting conflicting systematic review evidence.World J Gastrointest Pharmacol Ther. 2017 May 6;8(2):99-102. doi: 10.4292/wjgpt.v8.i2.99. World J Gastrointest Pharmacol Ther. 2017. PMID: 28533918 Free PMC article.
References
References to studies included in this review
Crohn's III 1997 {published data only}
-
- Hanauer SB, Stromberg U. Oral Pentasa in the treatment of active Crohn's disease: A meta‐analysis of double‐blind, placebo‐controlled trials. Clinical Gastroenterology and Hepatology 2004;2(5):379‐88. - PubMed
-
- Hoechst Marion Roussel Inc. Clinical study report: efficacy and safety of oral Pentasa in the treatment of active Crohn's disease (Crohn's III). January 28, 1997.
Gross 1995 {published data only}
-
- Gross V, Andus T, Fischbach W, Weber A, Gierend M, Hartmann F, et al. Comparison between high dose 5‐aminosalicylic acid and 6‐methylprednisolone in active Crohn's ileocolitis. A multicenter randomized double‐blind study. German 5‐ASA Study Group. Zeitschrift fur Gastroenterologie 1995;33(10):581‐4. - PubMed
Mahida 1990 {published data only}
-
- Mahida YR, Jewell DP. Slow‐release 5‐amino‐salicylic acid (Pentasa) for the treatment of active Crohn's disease. Digestion 1990;45(2):88‐92. - PubMed
Maier 1985 {published data only}
-
- Maier K, Fruhmorgen P, Bode JC, Heller T, Gaisberg U, Klotz U. Successful acute treatment of chronic inflammatory intestinal diseases with oral 5‐aminosalicylic acid. Deutsche Medizinische Wochenschrift 1985;110(10):363‐8. - PubMed
Maier 1990 {published data only}
-
- Maier K, Frick HJ, Gaisberg U, Teufel TH, Klotz U. Clinical efficacy of oral mesalamine in Crohn's disease. Can J Gastroenterol 1990;4(1):13‐8.
Malchow 1984 {published data only}
-
- Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, et al. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology 1984;86(2):249‐66. - PubMed
Martin 1990 {published data only}
-
- Martin F, Sutherland L, Beck IT, Anderson AH, Williams CN, Saibil F, et al. Oral 5‐ASA versus prednisolone in short term treatment of Crohn's disease: A multicentre controlled trial. Canadian Journal of Gastroenterology 1990;4(7):452‐7.
Prantera 1999 {published data only}
-
- Prantera C, Cottone M, Pallone F, Annese V, Franze A, Cerutti R, et al. Mesalamine in the treatment of mild to moderate active Crohn's ileitis: results of a randomized, multicenter trial. Gastroenterology 1999;116(3):521‐6. - PubMed
Rasmussen 1987 {published data only}
-
- Rasmussen SN, Lauritsen K, Tage‐Jensen U, Nielsen OH, Bytzer P, Jacobsen O, et al. 5‐Aminosalicylic acid in the treatment of Crohn's disease. A 16‐week double‐blind, placebo‐controlled, multicentre study with Pentasa. Scandinavian Journal of Gastroenterology 1987;22(7):877‐83. - PubMed
Rijk 1991 {published data only}
-
- Rijk MC, Hogezand RA, Lier HJ, Tongeren JH. Sulphasalazine and prednisone compared with sulphasalazine for treating active Crohn disease. A double‐blind, randomized, multicenter trial. Annals of Internal Medicine 1991;114(6):445‐50. - PubMed
Saverymuttu 1986 {published data only}
-
- Saverymuttu SH, Gupta S, Keshavarzian A, Donovan B, Hodgson HJ. Effect of a slow‐release 5'‐aminosalicylic acid preparation on disease activity in Crohn's disease. Digestion 1986;33(2):89‐91. - PubMed
Scholmerich 1990 {published data only}
-
- Jenss H, Hartmann F, Schölmerich J, und Deutsche 5‐ASA Studiengruppe. 5‐Aminosalicylic acid versus methylprednisolone in the therapy of acute Crohn's disease ‐ results of a double‐blind multicentric clinical study [5‐aminosalicylsäure versus methylprednisolon in der therapie des aktiven morbus Crohn ‐ ergebnisse einer doppelblinden multizentrischen klinischen studie]. Klinische Wochenschrift 1990;68(Suppl 19):34.
-
- Scholmerich J, Jenss H, Hartmann F, Dopfer H. Oral 5‐aminosalicylic acid versus 6‐methylprednisolone in active Crohn's disease. Canadian Journal of Gastroenterology 1990;4(7):446‐51.
Singleton 1993 {published data only}
-
- Singleton JW, Hanauer S, Robinson M. Quality‐of‐life results of double‐blind, placebo‐controlled trial of mesalamine in patients with Crohn's disease. Digestive Diseases and Sciences 1995;40(5):931‐5. - PubMed
-
- Singleton JW, Hanauer SB, Gitnick GL, Peppercorn MA, Robinson MG, Wruble LD, et al. Mesalamine capsules for the treatment of active Crohn's disease: results of a 16‐week trial. Pentasa Crohn's Disease Study Group. Gastroenterology 1993;104(5):1293‐301. - PubMed
Singleton 1994 {published data only}
-
- Nordic Research Inc. Clinical research report: efficacy and safety of oral Pentasa in the treatment of active Crohn's disease (Crohn's II). October 23, 1991.
-
- Singleton J. Second trial of mesalamine therapy in the treatment of active Crohn's disease. Gastroenterology 1994;107(2):632‐3. - PubMed
Summers 1979 {published data only}
-
- Singleton JW. Results of treatment with sulfasalazine in the American Multicenter Study on the treatment of Crohn disease (National Cooperative Crohn's Disease Study) [Ergebnisse der behandlung mit sulfasalazine der amerikanischen multcienter‐studie zur behandlung des morbus Crohn (National Cooperative Crohn's Disease Study [NCCDS])]. Zeitschrift fur Gastroenterologie ‐ Verhandlungsband 1981 Jun;19:38‐40. - PubMed
-
- Singleton JW, Law DH, Kelley ML Jr, Mekhjian HS, Sturdevant RA. National Cooperative Crohn's Disease Study: adverse reactions to study drugs. Gastroenterology 1979;77(4 Pt 2):870‐82. - PubMed
-
- Summers RW, Singleton JW. National Cooperative Crohn's Disease Study (NCCDS): a controlled prospective trial of three drugs vs placebo. Gut 1977;18(11):A972‐3.
-
- Summers RW, Switz DM, Sessions JT Jr, Becktel JM, Best WR, Kern F Jr, et al. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology 1979;77(4 Pt 2):847‐69. - PubMed
Thomsen 1998 {published data only}
-
- Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, et al. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide‐Mesalamine Study Group. New England Journal of Medicine 1998;339(6):370‐4. - PubMed
-
- Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, et al. Budesonide and mesalazine in active Crohn's disease: a comparison of the effects on quality of life. American Journal of Gastroenterology 2002 Mar;97(3):649‐53. - PubMed
Tremaine 1994 {published data only}
-
- Tremaine WJ, Schroeder KW, Harrison JM, Zinsmeister AR. A randomized, double‐blind, placebo‐controlled trial of the oral mesalamine (5‐ASA) preparation, Asacol, in the treatment of symptomatic Crohn's colitis and ileocolitis. Journal of Clinical Gastroenterology 1994;19(4):278‐82. - PubMed
Tromm 2011 {published data only}
-
- Tromm A, Bungani I, Tomsová E, Tulassay Z, Luká M, Kykal J, Bátovský M, Fixa B, Gabalec L, Safadi R, Kramm H, J.Altorjay I, Löhr H, Koutroubakis I, Bar‐Meir S, Stimac D, Schäffeler E, Glasmacher C, Dilger K, Mohrbacher R, Greinwald R. Budesonide 9 mg is at least as effective as mesalamine 4.5 g in patients with mildly to moderately active Crohn's disease. Gastroenterology 2011;140(2):425‐34. - PubMed
-
- Tromm A, Bunganic I, Tomsová E, Tulassay Z, Lukas M, Kykal J, et al. Both budesonide (9mg) as well as high‐dose eudragit‐l‐coated mesalamine(4.5g) lead to high remission rates in moderately active Crohn's disease patients: a double‐blind, double‐dummy, randomized, multicenter study. Gastroenterology 2009;136(5 supplement 1):A65.
Van Hees 1981 {published data only}
-
- Hees P, Velde G, Hogezand R, Driessen W, Bakker J, Lier H, et al. Effect of sulphasalazine in patients with Crohn's disease. a controlled double‐blind trial. Hepato‐Gastroenterology 1980;27(SUPPL.):E38.5.
-
- van HeesP, Hogezand R, Velde G. Effect of sulphasalazine in patients with active Crohn's disease. a controlled double‐blind trial. Netherlands Journal of Medicine 1981;24(4):157.
Wright 1995 {published data only}
-
- Wright JP, Jewell DP, Modigliani R, Malchow H. A randomized, double‐blind, placebo‐controlled trial of olsalazine for active Crohn's disease. Inflammatory Bowel Diseases 1995;1(4):241‐6. - PubMed
References to studies excluded from this review
Anonymous 1985 {published data only}
-
- Salazopyrin in the management of Crohn's disease. The Japanese Research Committee for Crohn's disease. Gastroenterologia Japonica 1985;20(1):71‐81. - PubMed
Anonymous 1990 {published data only}
-
- Coated oral 5‐aminosalicylic acid versus placebo in maintaining remission of inactive Crohn's disease. International Mesalazine Study Group. Alimentary Pharmacology and Therapeutics 1990;4(1):55‐64. - PubMed
Anthonisen 1974 {published data only}
-
- Anthonisen P, Barany F, Folkenborg O, Holtz A, Jarnum S, Kristensen M, et al. The clinical effect of salazosulphapyridine (Salazopyrin r) in Crohn's disease. A controlled double‐blind study. Scandinavian Journal of Gastroenterology 1974;9(6):549‐54. - PubMed
-
- Anthonisen P, Bárány F, FolkenborgO, Holtz A, Jarnum S, KristensenM, et al. The clinical effect of salazosulphapyridine (Salazopyrin) in Crohn's disease. Report of a controlled double blind investigation. Ugeskrift for Laeger 1974;136(32):1798‐802. - PubMed
Arber 1995 {published data only}
-
- Arber N, Odes HS, Fireman Z, Lavie A, Broide E, Bujanover Y, et al. A controlled double blind multicenter study of the effectiveness of 5‐aminosalicylic acid in patients with Crohn's disease in remission. Journal of Clinical Gastroenterology 1995;20(3):203‐6. - PubMed
Ardizzone 2004 {published data only}
-
- Ardizzone S, Maconi G, Sampietro GM, Russo A, Radice E, Colombo E, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology 2004;127(3):730‐40. - PubMed
Beck 1988 {published data only}
-
- Beck IT, Hudacin J, Paterson WG, Depew WT, Simon JB, Groll A. Mesalazine in the treatment of active Crohn's disease. Canadian Journal of Gastroenterology 1988;2(Suppl A):63A‐70A.
Bergman 1976 {published data only}
-
- Bergman L, Krause U. Postoperative treatment with corticosteroids and salazosulphapyridine (Salazopyrin) after radical resection for Crohn's disease. Scandinavian Journal of Gastroenterology 1976;11(7):651‐6. - PubMed
Blichfeldt 1978 {published data only}
-
- Blichfeldt P, Blomhoff JP, Myhre E, Gjone E. Metronidazole in Crohn's disease. A double blind cross‐over clinical trial. Scandinavian Journal of Gastroenterology 1978;13(1):123‐7. - PubMed
Bresci 1994 {published data only}
-
- Bresci G, Parisi G, Banti S. Long‐term therapy with 5‐aminosalicylic acid in Crohn's disease: is it useful? Our four years experience. International Journal of Clinical Pharmacology Research 1994;14(4):133‐8. - PubMed
Brignola 1992 {published data only}
-
- Brignola C, Iannone P, Pasquali S, Campieri M, Gionchetti P, Belluzzi A, et al. Placebo‐controlled trial of oral 5‐ASA in relapse prevention of Crohn's disease. Digestive Diseases and Sciences 1992;37(1):29‐32. - PubMed
Brignola 1995 {published data only}
-
- Brignola C, Cottone M, Pera A, Ardizzone S, Scribano ML, Franchis R, et al. Mesalamine in the prevention of endoscopic recurrence after intestinal resection for Crohn's disease. Italian Cooperative Study Group. Gastroenterology 1995;108(2):345‐9. - PubMed
Caprilli 1994 {published data only}
-
- Caprilli R, Andreoli A, Capurso L, Corrao G, D'Albasio G, Gioieni A, et al. Oral mesalazine (5‐aminosalicylic acid; Asacol) for the prevention of post‐operative recurrence of Crohn's disease. Gruppo Italiano per lo Studio del Colon e del Retto (GISC). Alimentary Pharmacology and Therapeutics 1994;8(1):35‐43. - PubMed
Caprilli 2003 {published data only}
-
- Caprilli R, Cottone M, Tonelli F, Sturniolo G, Castiglione F, Annese V, et al. Two mesalazine regimens in the prevention of the post‐operative recurrence of Crohn's disease: a pragmatic, double‐blind, randomized controlled trial. Alimentary Pharmacology and Therapeutics 2003;17(4):517‐23. - PubMed
Cezard 2009 {published data only}
-
- Cezard JP, Munck A, Mouterde O, Morali A, Lenaerts C, Lachaux A, et al. Prevention of relapse by mesalazine (Pentasa) in pediatric Crohn's disease: a multicenter, double‐blind, randomized, placebo‐controlled trial. Gastroenterologie Clinique et Biologique 2009;33(1 pt 1):31‐40. - PubMed
Cohen 2000 {published data only}
-
- Cohen Z. Crohn's disease ‐ Outcome of surgery. Drugs of Today 2000;36(Suppl G):51‐7.
Colombel 1999 {published data only}
-
- Colombel JF, Lemann M, Cassagnou M, Bouhnik Y, Duclos B, Dupas JL, et al. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn's disease. Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). American Journal of Gastroenterology 1999;94(3):674‐8. - PubMed
de Franchis 1997 {published data only}
-
- Franchis R, Omodei P, Ranzi T, Brignola C, Rocca R, Prada A, et al. Controlled trial of oral 5‐aminosalicylic acid for the prevention of early relapse in Crohn's disease. Alimentary Pharmacology and Therapeutics 1997;11(5):845‐52. - PubMed
Del Corso 1995 {published data only}
-
- Corso L, Moruzzo D, Romanelli AM, Norpoth M, Pentimone F, Bresci G. [5‐Aminosalicylic acid in the prevention of recurrences of Crohn's disease]. Deutsche Medizinische Wochenschrif 1995;120(50):1723‐7. - PubMed
Dirks 1989 {published data only}
-
- Dirks E, Goebell H, Schaarschmidt K, Forster S, Quebe‐Fehling E, Eigler FW. Clinical relapse of Crohn's disease under standardized conservative treatment and after excisional surgery. Digestive Diseases and Sciences 1989;34(12):1832‐40. - PubMed
Ewe 1976 {published data only}
-
- Ewe K, Holtermuller KH, Baas U, Eckhart V, Krieg H, Kutzner J, et al. [Prevention of recurrence by salazosulfapyridine (azulfidine) therapy in Crohn's disease. A double blind study]. Verhandlungen der Deutschen Gesellschaft fur Innere Medizin 1976;82 Pt 1:930‐2. - PubMed
Ewe 1984 {published data only}
-
- Ewe K, Malchow H, Herfarth, C. Radical operation and recurrence prevention with azulfidine in Crohn disease: a prospective multicenter study‐‐initial results [Operative Radikalitat und Rezidivprophylaxe mit Azulfidine bei M. Crohn: Eine prospektive multizentrische Studie‐‐Erste Ergebnisse.]. Langenbecks Archiv für Chirurgie 1984;364:427‐30. - PubMed
Ewe 1986 {published data only}
-
- Ewe K, Herfarth Ch, Malchow H, Jesdinsky H. Crohn study II: Influence of surgical radicality and salazosulfapyridine prophylaxis on postoperative relapses. Klinische Wochenschrift 1986;64:50‐1.
Ewe 1989 {published data only}
-
- Ewe K, Herfarth C, Malchow H, Jesdinsky HJ. Postoperative recurrence of Crohn's disease in relation to radicality of operation and sulfasalazine prophylaxis: a multicenter trial. Digestion 1989;42(4):224‐32. - PubMed
Fiasse 1990 {published data only}
-
- Fiasse R, Fontaine F, Heuverzwijn R, Biemans M. Prevention of Crohn's disease recurrences after intestinal resection with eudragid‐l‐coated 5‐aminosalicylic acid. An interim report of a one year double‐blind placebo controlled study (ABSTRACT). Acta Gastroenterologica Belgica 1990;53(1):A11.
Florent 1996 {published data only}
-
- Florent C, Cortot A, Quandale P, Sahmound T, Modigliani R, Sarfaty E, et al. Placebo‐controlled clinical trial of mesalazine in the prevention of early endoscopic recurrences after resection for Crohn's disease. Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). European Journal of Gastroenterology and Hepatology 1996;8(3):229‐33. - PubMed
Gendre 1993 {published data only}
-
- Gendre JP, Mary JY, Florent C, Modigliani R, Colombel JF, Soule JC, et al. Oral mesalamine (Pentasa) as maintenance treatment in Crohn's disease: a multicenter placebo‐controlled study. The Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). Gastroenterology 1993;104(2):435‐9. - PubMed
Gerhardt 2001 {published data only}
-
- Gerhardt H, Seifert F, Buvari P, Vogelsang H, Repges R. [Therapy of active Crohn disease with Boswellia serrata extract H 15]. Zeitschrift fur Gastroenterologie 2001;39(1):11‐7. - PubMed
Goldstein 1987 {published data only}
-
- Goldstein F, Farquhar S, Thornton JJ, Abramson J. Favorable effects of sulfasalazine on small bowel Crohn's disease: a long‐term study.". American Journal of Gastroenterology 1987;82(9):848‐853. - PubMed
Griffiths 1993 {published data only}
-
- Griffiths A, Koletzko S, Sylvester F, Marcon M, Sherman P. Slow‐release 5‐aminosalicylic acid therapy in children with small intestinal Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 1993;17(2):186‐92. - PubMed
Guslandi 2000 {published data only}
-
- Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Digestive Diseases and Sciences 2000;45(7):1462‐4. - PubMed
Hanauer 1993 {published data only}
-
- Hanauer SB, Krawitt EL, Robinson M, Rick GG, Safdi MA. Long‐term management of Crohn's disease with mesalamine capsules (Pentasa). Pentasa Crohn's Disease Compassionate Use Study Group. American Journal of Gastroenterology 1993;88(9):1343‐51. - PubMed
Hanauer 2004b {published data only}
-
- Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, et al. Postoperative maintenance of Crohn's disease remission with 6‐mercaptopurine, mesalamine, or placebo: a 2‐year trial. Gastroenterology 2004;127(3):723‐9. - PubMed
Howaldt 1993 {published data only}
-
- Howaldt S, Raedler A, Reinecker HC, Berghaus D, Hoyer S, Kaiser B, et al. Comparative trial of remission prophylaxis in quiescent Crohn's disease with oral 4‐aminosalicylic acid versus 5‐aminosalicylic acid slow release tablets. Can J Gastroenterol 1993;7(2):241‐4.
Klein 1995 {published data only}
-
- Klein O, Colombel JF, Lescut D, Gambiez L, Desreumaux P, Quandalle P, et al. Remaining small bowel endoscopic lesions at surgery have no influence on early anastomotic recurrences in Crohn's disease. Am J Gastroenterol 1995;90(11):1949‐52. - PubMed
Klotz 1980 {published data only}
-
- Klotz U, Maier K, Fischer C, Heinkel K. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn's disease. New England Journal of Medicine 1980;303(26):1499‐502. - PubMed
Lennard‐Jones 1977 {published data only}
Lichtenstein 2009a {published data only}
-
- Lichtenstein GR, Ramsey D. Experience with delayed‐release mesalamine for maintenance of remission of Crohn's disease (CD). Gastroenterology 2009;136(5):A661‐2.
Lichtenstein 2009b {published data only}
-
- Lichtenstein GR, Ramsey D. Experience with delayed‐release mesalamine for active Crohn's disease (CD). Gastroenterology 2009;136(5):A657‐8.
Lochs 1991 {published data only}
-
- Lochs H, Steinhardt HJ, Klaus‐Wentz B, Zeitz M, Vogelsang H, Sommer H, et al. Comparison of enteral nutrition and drug treatment in active Crohn's disease. Results of the European Cooperative Crohn's Disease Study. IV. Gastroenterology 1991;101(4):881‐8. - PubMed
Lochs 2000 {published data only}
-
- Lochs H, Mayer M, Fleig WE, Mortensen PB, Bauer P, Genser D, et al. Prophylaxis of postoperative relapse in Crohn's disease with mesalamine: European Cooperative Crohn's Disease Study VI. Gastroenterology 2000;118(2):264‐73. - PubMed
Mahmud 2001 {published data only}
Malchow 1990 {published data only}
-
- Malchow H, Steinhardt HJ, Lorenz‐Meyer H, Strohm WD, Rasmussen S, Sommer H, et al. Feasibility and effectiveness of a defined‐formula diet regimen in treating active Crohn's disease. European Cooperative Crohn's Disease Study III. Scandinavian Journal of Gastroenterology 1990;25(3):235‐44. - PubMed
Mantzaris 2003 {published data only}
-
- Mantzaris GJ, Petraki K, Sfakianakis M, Archavlis E, Christidou A, Chadio‐Iordanides H, et al. Budesonide versus mesalamine for maintaining remission in patients refusing other immunomodulators for steroid‐dependent Crohn's disease. Clinical Gastroenterology and Hepatology 2003;1(2):122‐8. - PubMed
Mate‐Jimenez 2000 {published data only}
-
- Mate‐Jimenez J, Hermida C, Cantero‐Perona J, Moreno‐Otero R. 6‐mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid‐dependent inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2000;12(11):1227‐33. - PubMed
McLeod 1995 {published data only}
-
- McLeod RS, Wolff BG, Steinhart AH, Carryer PW, O'Rourke K, Andrews DF, et al. Prophylactic mesalamine treatment decreases postoperative recurrence of Crohn's disease. Gastroenterology 1995;109(2):404‐13. - PubMed
Modigliani 1996 {published data only}
-
- Modigliani R, Colombel JF, Dupas JL, Dapoigny M, Costil V, Veyrac M, et al. Mesalamine in Crohn's disease with steroid‐induced remission: effect on steroid withdrawal and remission maintenance, Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives. Gastroenterology 1996;110(3):688‐93. - PubMed
Orlando 2012 {published data only}
-
- Orlando A, Mocciaro F, Scimeca D, Rispo A, Scribano ML, Testa A, et al. Early post‐operative endoscopic recurrence in crohn's disease in a large prospective Italian multicenter cohort. Digestive and Liver Disease 2012;44:S81.
Papi 2009 {published data only}
-
- Papi C, Aratari A, Tornatore V, Koch M, Capurso L, Caprilli, R. Long‐term prevention of post‐operative recurrence in Crohn's disease cannot be affected by mesalazine. Journal of Crohn's and Colitis 2009;3(2):109‐14. - PubMed
Prantera 1992 {published data only}
-
- Prantera C, Pallone F, Brunetti G, Cottone M, Miglioli M. Oral 5‐aminosalicylic acid (Asacol) in the maintenance treatment of Crohn's disease. The Italian IBD Study Group. Gastroenterology 1992;103(2):363‐8. - PubMed
Rasmussen 1983 {published data only}
-
- Rasmussen SN, Binder V, Maier K, Bondesen S, Fischer C, Klotz U, et al. Treatment of Crohn's disease with peroral 5‐aminosalicylic acid. Gastroenterology 1983;85(6):1350‐3. - PubMed
Reinisch 2010 {published data only}
-
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar‐Meir S, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomised, double‐blind, double‐dummy, multicentre trial. Gut 2010;59(6):752‐9. - PubMed
Romano 2005 {published data only}
-
- Romano C, Cucchiara S, Barabino A, Annese V, Sferlazzas C. Usefulness of omega‐3 fatty acid supplementation in addition to mesalazine in maintaining remission in pediatric Crohn's disease: a double‐blind, randomized, placebo‐controlled study. World Journal of Gastroenterology 2005;11(45):7118‐21. - PMC - PubMed
Rosen, Ursing 1982 {published data only}
-
- Rosen A, Ursing B, Alm T, Barany F, Bergelin I, Ganrot‐Norlin K, et al. A comparative study of metronidazole and sulfasalazine for active Crohn's disease: the cooperative Crohn's disease study in Sweden. I. Design and methodologic considerations. Gastroenterology 1982;83(3):541‐9. - PubMed
-
- Ursing B, Alm T, Barany F, Bergelin I, Ganrot‐Norlin K, Hoevels J, et al. A comparative study of metronidazole and sulfasalazine for active Crohn's disease: the cooperative Crohn's disease study in Sweden. II. Result. Gastroenterology 1982;83(3):550‐62. - PubMed
Savarino 2013 {published data only}
-
- Savarino E, Bodini G, Dulbecco P, Marabotto E, Assandri L, Bruzzone L, Mazza F, Fazio V, Giambruno E, Gemignani L, Savarino V. Adalimumab Is More Effective Than Azathioprine and Mesalamine At Preventing Postoperative Recurrence of Crohn's Disease ‐ A Randomized Trial. Gastroenterology 2013;144(5):S21. - PubMed
Schneider 1985 {published data only}
-
- Schneider MU, Laudage G, Guggenmoos‐Holzmann I, Riemann JF. [Metronidazole in the treatment of Crohn disease. Results of a controlled randomized prospective study]. Deutsche Medizinische Wochenschrift 1985;110(45):1724‐30. - PubMed
Schreiber 1994 {published data only}
Singleton 1979 {published data only}
-
- Singleton JW, Summers RW, Kern F Jr, Becktel JM, Best WR, Hansen RN, et al. A trial of sulfasalazine as adjunctive therapy in Crohn's disease. Gastroenterology 1979;77(4 Pt2):887‐97. - PubMed
Stober 1983 {published data only}
-
- Stober B, Nutzenadel W, Ullrich F. [Basic diet in Crohn's disease]. Monatsschr Kinderheilkd 1983;131(10):721‐4. - PubMed
Sutherland 1997 {published data only}
-
- Sutherland LR, Martin F, Bailey RJ, Fedorak RN, Poleski M, Dallaire C, et al. A randomized, placebo‐controlled, double‐blind trial of mesalamine in the maintenance of remission of Crohn's disease. The Canadian Mesalamine for Remission of Crohn's Disease Study Group. Gastroenterology 1997;112(4):1069‐77. - PubMed
Tao 2009 {published data only}
Terranova 2001 {published data only}
-
- Terranova M, Leonello G, Macri A, Versaci A, Scuderi G, Crisafulli C, et al. Enteral nutrition in the short‐term treatment of active inflammatory bowel diseases: A single‐centre experience. Rivista Italiana di Nutrizione Parenterale ed Enterale 2001;19(1):12‐7.
Terrin 2002 {published data only}
-
- Terrin G, Canani RB, Ambrosini A, Viola F, Mesquita MB, Nardo G, Dito L, Cucchiara S. A semielemental diet (Pregomin) as primary therapy for inducing remission in children with active Crohn's disease. Italian Journal of Pediatrics 2002;25(5):401‐5.
Thomson 1995 {published data only}
-
- Thomson AB, Wright JP, Vatn M, Bailey RJ, Rachmilewitz D, Adler M, et al. Mesalazine (Mesasal/Claversal) 1.5 g b.d. vs. placebo in the maintenance of remission of patients with Crohn's disease. Alimentary Pharmacology and Therapeutics 1995;9(6):673‐83. - PubMed
Triantafillidis 2010 {published data only}
-
- Triantafillidis JK, Stamataki A, Karagianni V, Gikas A, Malgarinos G. Maintenance treatment of Crohn's disease with a polymeric feed rich in TGF‐beta. Annals of Gastroenterology 2010;23(2):113‐8.
Wellmann 1986 {published data only}
Wellmann 1988 {published data only}
-
- Wellmann W, Schroder U. New oral preparations for maintenance therapy in Crohn's disease (abstract). Canadian Journal of Gastroenterology 1988;2(Supplement A):71A‐2.
Wenckert 1978 {published data only}
-
- Wenckert A, Kristensen M, Eklund AE, Barany F, Jarnum S, Worning H, et al. The long‐term prophylactic effect of salazosulphapyridine (Salazopyrin) in primarily resected patients with Crohn's disease. A controlled double‐blind trial. Scand J Gastroenterol 1978;13(2):161‐7. - PubMed
Yamamoto 2009 {published data only}
-
- Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn's disease: a prospective pilot study. Inflammatory Bowel Diseases 2009;15(10):1460‐6. - PubMed
Additional references
Azad 1977
-
- Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet 1977;2(8044):892‐5. - PubMed
Bar‐Meir 1998
-
- Bar‐Meir S, Chowers Y, Lavy A, Abramovitch D, Sternberg A, Leichtmann G, et al. Budesonide versus prednisone in the treatment of active Crohn's disease. The Israeli Budesonide Study Group. Gastroenterology 1998;115(4):835‐40. - PubMed
Binder 1982
-
- Binder V, Both H, Hansen PK, Hendriksen C, Kreiner S, Torp‐Pedersen K. Incidence and prevalence of ulcerative colitis and Crohn's disease in the County of Copenhagen, 1962 to 1978. Gastroenterology 1982;83(3):563‐8. - PubMed
Brant 1999
-
- Brant R, Sutherland L, Hilsden R. Examining the minimum important difference. Statistics in Medicine 1999;18(19):2593‐603. - PubMed
Campieri 1997
Cellier 1994
-
- Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 1994;35(2):231‐5. - PMC - PubMed
De Dombal 1974
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dubuquoy 2006
Feagan 2004
-
- Feagan BG. 5‐ASA therapy for active Crohn's disease: old friends, old data, and a new conclusion. Clinical Gastroenterology and Hepatology 2004;2(5):376‐8. - PubMed
Gross 1996
-
- Gross V, Andus T, Caesar I, Bischoff SC, Lochs H, Tromm A, et al. Oral pH‐modified release budesonide versus 6‐methylprednisolone in active Crohn's disease. German/Austrian Budesonide Study Group. European Journal of Gastroenterology and Hepatology 1996;8(9):905‐9. - PubMed
Guyatt 2008
Hanauer 2001
-
- Hanauer SB, Sandborn W. Management of Crohn's disease in adults. American Journal of Gastroenterology 2001;96(3):635‐43. - PubMed
Hanauer 2003
-
- Hanauer SB, Present DH. The state of the art in the management of inflammatory bowel disease. Reviews in Gastroenterological Disorders 2003;3(2):81‐92. - PubMed
Hanauer 2004
-
- Hanauer SB, Stromberg U. Oral Pentasa in the treatment of active Crohn's disease: A meta‐analysis of double‐blind, placebo‐controlled trials. Clinical Gastroenterology and Hepatology 2004;2(5):379‐88. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors(s) editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Levesque 2012
-
- Levesque BG, Sandborn WJ. Setting a high threshold for noninferiority: mesalamine and budesonide in Crohn's disease. Inflammatory Bowel Diseases 2012;18(4):795‐6. - PubMed
Loftus 2004
-
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence and environmental influences. Gastroenterology 2004;126(6):1504‐17. - PubMed
MacDermott 2000
-
- MacDermott RP. Progress in understanding the mechanisms of action of 5‐aminosalicylic acid. American Journal of Gastroenterology 2000;95(12):3343‐5. - PubMed
Munafo 2004
-
- Munafo MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta‐analysis. Psychiatry Research 2004;129(1):39‐44. - PubMed
Rao 1987
Rousseaux 2005
Rutgeerts 1994
-
- Rutgeerts P, Lofberg R, Malchow H, Lamers C, Olaison G, Jewell D, et al. A comparison of budesonide with prednisolone for active Crohn's disease. New England Journal of Medicine 1994;331(13):842‐5. - PubMed
Sandborn 2002a
-
- Sandborn WJ. Rational selection of oral 5‐aminosalicylate formulations and prodrugs for the treatment of ulcerative colitis. American Journal of Gastroenterology 2002;97(12):2939‐41. - PubMed
Sandborn 2002b
-
- Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Lofberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology 2002;122(2):512‐30. - PubMed
Sandborn 2003
-
- Sandborn WJ, Feagan BG. Review article: mild to moderate Crohn's disease‐‐defining the basis for a new treatment algorithm. Alimentary Pharmacology and Therapeutics 2003;18(3):263‐77. - PubMed
Sandborn 2004
Schreiber 2005
-
- Schreiber S, Rutgeerts P, Fedorak RN, Khaliq‐Kareemi M, Kamm MA, Boivin M, et al. A randomized, placebo‐controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology 2005;129(3):807‐18. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s) editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Su 2004
-
- Su C, Lichtenstein GR, Krok K, Brensinger CM, Lewis JD. A meta‐analysis of the placebo rates of remission and response in clinical trials of active Crohn's disease. Gastroenterology 2004;126(5):1257‐69. - PubMed
Thomsen 2001
-
- Thomsen OO. A comparison of budesonide and mesalamine for active Crohn's disease. Correction. New England Journal of Medicine 2001;345(22):1652. - PubMed
Tvede 1983
-
- Tvede M, Bondesen S, Nielsen OH, Rasmussen SN. Serum antibodies to Bacteroides species in chronic inflammatory bowel disease. Scandinavian Journal of Gastroenterology 1983;18(6):783‐9. - PubMed
Wadworth 1991
-
- Wadworth AN, Fitton A. Olsalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in inflammatory bowel disease. Drugs 1991;41(4):647‐64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

