Use of complementary nucleobase-containing synthetic polymers to prepare complex self-assembled morphologies in water
- PMID: 27358655
- PMCID: PMC4894073
- DOI: 10.1039/c6py00263c
Use of complementary nucleobase-containing synthetic polymers to prepare complex self-assembled morphologies in water
Abstract
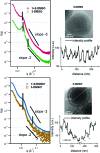
Amphiphilic nucleobase-containing block copolymers with poly(oligo(ethylene glycol) methyl ether methacrylate) as the hydrophilic block and nucleobase-containing blocks as the hydrophobic segments were successfully synthesized using RAFT polymerization and then self-assembled via solvent switch in aqueous solutions. Effects of the common solvent on the resultant morphologies of the adenine (A) and thymine (T) homopolymers, and A/T copolymer blocks and blends were investigated. These studies highlighted that depending on the identity of the common solvent, DMF or DMSO, spherical micelles or bicontinuous micelles were obtained. We propose that this is due to the presence of A-T interactions playing a key role in the morphology and stability of the resultant nanoparticles, which resulted in a distinct system compared to individual adenine or thymine polymers. Finally, the effects of annealing on the self-assemblies were explored. It was found that annealing could lead to better-defined spherical micelles and induce a morphology transition from bicontinuous micelles to onion-like vesicles, which was considered to occur due to a structural rearrangement of complementary nucleobase interactions resulting from the annealing process.
Figures





Similar articles
-
Block Copolymer Self-assembly: Exploitation of Hydrogen Bonding for Nanoparticle Morphology Control via Incorporation of Triazine Based Comonomers by RAFT Polymerization.Small. 2024 Oct;20(40):e2401129. doi: 10.1002/smll.202401129. Epub 2024 Jun 4. Small. 2024. PMID: 38837298
-
Size effects of self-assembled block copolymer spherical micelles and vesicles on cellular uptake in human colon carcinoma cells.J Mater Chem B. 2014 May 21;2(19):2883-2891. doi: 10.1039/c3tb21751e. Epub 2014 Apr 7. J Mater Chem B. 2014. PMID: 32261483
-
Self-Aggregation in Aqueous Media of Amphiphilic Diblock and Random Block Copolymers Composed of Monomers with Long Side Chains.Langmuir. 2023 Mar 7;39(9):3380-3390. doi: 10.1021/acs.langmuir.2c03294. Epub 2023 Feb 21. Langmuir. 2023. PMID: 36802652
-
Spontaneous generation of amphiphilic block copolymer micelles with multiple morphologies through interfacial Instabilities.J Am Chem Soc. 2008 Jun 11;130(23):7496-502. doi: 10.1021/ja801268e. Epub 2008 May 15. J Am Chem Soc. 2008. PMID: 18479130
-
Controlled Self-Assembly of Amphiphilic Random Copolymers into Folded Micelles and Nanostructure Materials.J Oleo Sci. 2020 Jun 9;69(6):529-538. doi: 10.5650/jos.ess20089. Epub 2020 May 14. J Oleo Sci. 2020. PMID: 32404554 Review.
Cited by
-
Nucleobase-Containing Polymers: Structure, Synthesis, and Applications.Polymers (Basel). 2017 Dec 1;9(12):666. doi: 10.3390/polym9120666. Polymers (Basel). 2017. PMID: 30965964 Free PMC article. Review.
-
Microstructure-Thermal Property Relationships of Poly (Ethylene Glycol-b-Caprolactone) Copolymers and Their Micelles.Polymers (Basel). 2022 Oct 16;14(20):4365. doi: 10.3390/polym14204365. Polymers (Basel). 2022. PMID: 36297943 Free PMC article.
-
Synthetic Approaches for Copolymers Containing Nucleic Acids and Analogues: Challenges and Opportunities.Polym Chem. 2021 Apr 21;12(15):2193-2204. doi: 10.1039/d0py01707h. Epub 2021 Mar 29. Polym Chem. 2021. PMID: 34394751 Free PMC article.
-
Bioinspired and biomimetic nucleobase-containing polymers: the effect of selective multiple hydrogen bonds.Chem Sci. 2024 Nov 5;15(45):18698-18714. doi: 10.1039/d4sc06720g. eCollection 2024 Nov 20. Chem Sci. 2024. PMID: 39568625 Free PMC article. Review.
-
Controlled node growth on the surface of polymersomes.Chem Sci. 2024 Feb 16;15(12):4396-4402. doi: 10.1039/d3sc05915d. eCollection 2024 Mar 20. Chem Sci. 2024. PMID: 38516085 Free PMC article.
References
-
- Khan A., Haddleton D. M., Hannon M. J., Kukulj D., Marsh A. Macromolecules. 1999;32:6560–6564.
-
- South C. R., Weck M. Macromolecules. 2007;40:1386–1394.
-
- Lo P. K., Sleiman H. F. J. Am. Chem. Soc. 2009;131:4182–4183. - PubMed
-
- McHale R., Patterson J. P., Zetterlund P. B., O'Reilly R. K. Nat. Chem. 2012;4:491–497. - PubMed
-
- Ilhan F., Galow T. H., Gray M., Clavier G., Rotello V. M. J. Am. Chem. Soc. 2000;122:5895–5896.
LinkOut - more resources
Full Text Sources
Other Literature Sources
