A clinically authentic mouse model of enterovirus 71 (EV-A71)-induced neurogenic pulmonary oedema
- PMID: 27357918
- PMCID: PMC4928123
- DOI: 10.1038/srep28876
A clinically authentic mouse model of enterovirus 71 (EV-A71)-induced neurogenic pulmonary oedema
Abstract
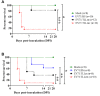
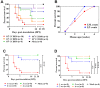
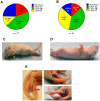
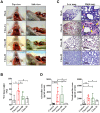
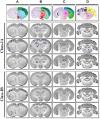
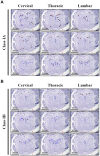
Enterovirus 71 (EV-A71) is a neurotropic virus that sporadically causes fatal neurologic illness among infected children. Animal models of EV-A71 infection exist, but they do not recapitulate in animals the spectrum of disease and pathology observed in fatal human cases. Specifically, neurogenic pulmonary oedema (NPE)-the main cause of EV-A71 infection-related mortality-is not observed in any of these models. This limits their utility in understanding viral pathogenesis of neurologic infections. We report the development of a mouse model of EV-A71 infection displaying NPE in severely affected animals. We inoculated one-week-old BALB/c mice with an adapted EV-A71 strain and identified clinical signs consistent with observations in human cases and other animal models. We also observed respiratory distress in some mice. At necropsy, we found their lungs to be heavier and incompletely collapsed compared to other mice. Serum levels of catecholamines and histopathology of lung and brain tissues of these mice strongly indicated onset of NPE. The localization of virally-induced brain lesions also suggested a potential pathogenic mechanism for EV-A71-induced NPE. This novel mouse model of virally-induced NPE represents a valuable resource for studying viral mechanisms of neuro-pathogenesis and pre-clinical testing of potential therapeutics and prophylactics against EV-A71-related neurologic complications.
Figures
Similar articles
-
Intra-host emergence of an enterovirus A71 variant with enhanced PSGL1 usage and neurovirulence.Emerg Microbes Infect. 2019;8(1):1076-1085. doi: 10.1080/22221751.2019.1644142. Emerg Microbes Infect. 2019. PMID: 31339457 Free PMC article.
-
Pulmonary and central nervous system pathology in fatal cases of hand foot and mouth disease caused by enterovirus A71 infection.Pathology. 2016 Apr;48(3):267-74. doi: 10.1016/j.pathol.2015.12.450. Epub 2016 Mar 10. Pathology. 2016. PMID: 27020504
-
[Establishment of a Model of Infection by Enterovirus 71 in ICR Mice].Bing Du Xue Bao. 2016 Nov;32(6):671-82. Bing Du Xue Bao. 2016. PMID: 30004196 Chinese.
-
Recent advances in enterovirus A71 pathogenesis: a focus on fatal human enterovirus A71 infection.Arch Virol. 2022 Dec;167(12):2483-2501. doi: 10.1007/s00705-022-05606-4. Epub 2022 Sep 29. Arch Virol. 2022. PMID: 36171507 Review.
-
Immune responses against enterovirus A71 infection: Implications for vaccine success.Rev Med Virol. 2019 Sep;29(5):e2073. doi: 10.1002/rmv.2073. Epub 2019 Aug 1. Rev Med Virol. 2019. PMID: 31369184 Review.
Cited by
-
Intra-host emergence of an enterovirus A71 variant with enhanced PSGL1 usage and neurovirulence.Emerg Microbes Infect. 2019;8(1):1076-1085. doi: 10.1080/22221751.2019.1644142. Emerg Microbes Infect. 2019. PMID: 31339457 Free PMC article.
-
The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68) - what is the evidence for causation?Euro Surveill. 2018 Jan;23(3):17-00310. doi: 10.2807/1560-7917.ES.2018.23.3.17-00310. Euro Surveill. 2018. PMID: 29386095 Free PMC article. Review.
-
Pulmonary edema following central nervous system lesions induced by a non- mouse-adapted EV71 strain in neonatal BALB/c mice.Virol J. 2017 Dec 28;14(1):243. doi: 10.1186/s12985-017-0911-5. Virol J. 2017. PMID: 29282065 Free PMC article.
-
Translocator protein (TSPO) is a biomarker of Zika virus (ZIKV) infection-associated neuroinflammation.Emerg Microbes Infect. 2024 Dec;13(1):2348528. doi: 10.1080/22221751.2024.2348528. Epub 2024 May 27. Emerg Microbes Infect. 2024. PMID: 38662785 Free PMC article.
-
TSPO expression in a Zika virus murine infection model as an imaging target for acute infection-induced neuroinflammation.Eur J Nucl Med Mol Imaging. 2023 Feb;50(3):742-755. doi: 10.1007/s00259-022-06019-w. Epub 2022 Nov 9. Eur J Nucl Med Mol Imaging. 2023. PMID: 36348095 Free PMC article.
References
-
- Knowles N. J. et al.. In Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses (eds King A. M. Q., Adams M. J., Carstens E. B. & Lefkowitz E. J.) 855–880 (Elsevier, 2012).
-
- Wang S. M. et al.. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 29, 184–190, doi: 10.1086/520149 (1999). - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

