Activation of Wnt Signaling by Mechanical Loading Is Impaired in the Bone of Old Mice
- PMID: 27357062
- PMCID: PMC5397287
- DOI: 10.1002/jbmr.2900
Activation of Wnt Signaling by Mechanical Loading Is Impaired in the Bone of Old Mice
Abstract
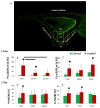
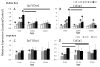
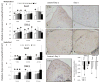
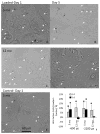
Aging diminishes bone formation engendered by mechanical loads, but the mechanism for this impairment remains unclear. Because Wnt signaling is required for optimal loading-induced bone formation, we hypothesized that aging impairs the load-induced activation of Wnt signaling. We analyzed dynamic histomorphometry of 5-month-old, 12-month-old, and 22-month-old C57Bl/6JN mice subjected to multiple days of tibial compression and corroborated an age-related decline in the periosteal loading response on day 5. Similarly, 1 day of loading increased periosteal and endocortical bone formation in young-adult (5-month-old) mice, but old (22-month-old) mice were unresponsive. These findings corroborated mRNA expression of genes related to bone formation and the Wnt pathway in tibias after loading. Multiple bouts (3 to 5 days) of loading upregulated bone formation-related genes, e.g., Osx and Col1a1, but older mice were significantly less responsive. Expression of Wnt negative regulators, Sost and Dkk1, was suppressed with a single day of loading in all mice, but suppression was sustained only in young-adult mice. Moreover, multiple days of loading repeatedly suppressed Sost and Dkk1 in young-adult, but not in old tibias. The age-dependent response to loading was further assessed by osteocyte staining for Sclerostin and LacZ in tibia of TOPGAL mice. After 1 day of loading, fewer osteocytes were Sclerostin-positive and, corroboratively, more osteocytes were LacZ-positive (Wnt active) in both 5-month-old and 12-month-old mice. However, although these changes were sustained after multiple days of loading in 5-month-old mice, they were not sustained in 12-month-old mice. Last, Wnt1 and Wnt7b were the most load-responsive of the 19 Wnt ligands. However, 4 hours after a single bout of loading, although their expression was upregulated threefold to 10-fold in young-adult mice, it was not altered in old mice. In conclusion, the reduced bone formation response of aged mice to loading may be due to failure to sustain Wnt activity with repeated loading. © 2016 American Society for Bone and Mineral Research.
Keywords: AGING; ANABOLIC; OSTEOBLAST; OSTEOCYTE; WNT/β-CATENIN SIGNALING.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures
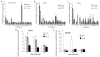
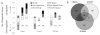
Similar articles
-
Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading.Bone. 2012 Jan;50(1):209-17. doi: 10.1016/j.bone.2011.10.025. Epub 2011 Oct 30. Bone. 2012. PMID: 22075208 Free PMC article.
-
Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered.Osteoporos Int. 2012 Apr;23(4):1225-34. doi: 10.1007/s00198-011-1656-4. Epub 2011 May 15. Osteoporos Int. 2012. PMID: 21573880 Free PMC article.
-
PTHrP-Derived Peptides Restore Bone Mass and Strength in Diabetic Mice: Additive Effect of Mechanical Loading.J Bone Miner Res. 2017 Mar;32(3):486-497. doi: 10.1002/jbmr.3007. Epub 2016 Oct 24. J Bone Miner Res. 2017. PMID: 27683064
-
Regulation of Wnt/β-catenin signaling within and from osteocytes.Bone. 2013 Jun;54(2):244-9. doi: 10.1016/j.bone.2013.02.022. Epub 2013 Mar 5. Bone. 2013. PMID: 23470835 Free PMC article. Review.
-
[Mechanical stress and Wnt signal].Clin Calcium. 2013 Jun;23(6):839-45. Clin Calcium. 2013. PMID: 23719496 Review. Japanese.
Cited by
-
The LINC Complex Assists the Nuclear Import of Mechanosensitive Transcriptional Regulators.Results Probl Cell Differ. 2022;70:315-337. doi: 10.1007/978-3-031-06573-6_11. Results Probl Cell Differ. 2022. PMID: 36348113
-
Osteocyte lacunar strain determination using multiscale finite element analysis.Bone Rep. 2020 May 19;12:100277. doi: 10.1016/j.bonr.2020.100277. eCollection 2020 Jun. Bone Rep. 2020. PMID: 32478144 Free PMC article.
-
Sost deficiency leads to reduced mechanical strains at the tibia midshaft in strain-matched in vivo loading experiments in mice.J R Soc Interface. 2018 Apr;15(141):20180012. doi: 10.1098/rsif.2018.0012. J R Soc Interface. 2018. PMID: 29669893 Free PMC article.
-
Osteoporosis treatments for intervertebral disc degeneration and back pain: a perspective.JBMR Plus. 2024 Apr 18;8(6):ziae048. doi: 10.1093/jbmrpl/ziae048. eCollection 2024 Jun. JBMR Plus. 2024. PMID: 38706880 Free PMC article.
-
Cancellous Bone May Have a Greater Adaptive Strain Threshold Than Cortical Bone.JBMR Plus. 2021 Mar 30;5(5):e10489. doi: 10.1002/jbm4.10489. eCollection 2021 May. JBMR Plus. 2021. PMID: 33977205 Free PMC article.
References
-
- Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res. 1978;26:13–7. - PubMed
-
- Parfitt AM, Villanueva AR, Foldes J, Rao DS. Relations between histologic indices of bone formation: implications for the pathogenesis of spinal osteoporosis. J Bone Miner Res. 1995;10(3):466–73. - PubMed
-
- Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33(3):387–98. - PubMed
-
- Rubin CT, Bain SD, McCleod KJ. Suppression of osteogenic response in the aging skeleton. Calcif Tissue Int. 1992;50(4):306–13. - PubMed
-
- Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res. 1995;10(10):1544–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

