Discovery of a Highly Selective Glycogen Synthase Kinase-3 Inhibitor (PF-04802367) That Modulates Tau Phosphorylation in the Brain: Translation for PET Neuroimaging
- PMID: 27355874
- PMCID: PMC4983481
- DOI: 10.1002/anie.201603797
Discovery of a Highly Selective Glycogen Synthase Kinase-3 Inhibitor (PF-04802367) That Modulates Tau Phosphorylation in the Brain: Translation for PET Neuroimaging
Abstract
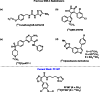
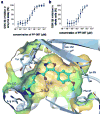
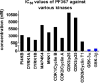
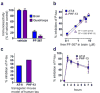
Glycogen synthase kinase-3 (GSK-3) regulates multiple cellular processes in diabetes, oncology, and neurology. N-(3-(1H-1,2,4-triazol-1-yl)propyl)-5-(3-chloro-4-methoxyphenyl)oxazole-4-carboxamide (PF-04802367 or PF-367) has been identified as a highly potent inhibitor, which is among the most selective antagonists of GSK-3 to date. Its efficacy was demonstrated in modulation of tau phosphorylation in vitro and in vivo. Whereas the kinetics of PF-367 binding in brain tissues are too fast for an effective therapeutic agent, the pharmacokinetic profile of PF-367 is ideal for discovery of radiopharmaceuticals for GSK-3 in the central nervous system. A (11) C-isotopologue of PF-367 was synthesized and preliminary PET imaging studies in non-human primates confirmed that we have overcome the two major obstacles for imaging GSK-3, namely, reasonable brain permeability and displaceable binding.
Keywords: Alzheimer's disease; glycogen synthase kinase-3; phosphorylation; positron emission tomography; tau proteins.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

Similar articles
-
Structural Basis for Achieving GSK-3β Inhibition with High Potency, Selectivity, and Brain Exposure for Positron Emission Tomography Imaging and Drug Discovery.J Med Chem. 2019 Nov 14;62(21):9600-9617. doi: 10.1021/acs.jmedchem.9b01030. Epub 2019 Oct 21. J Med Chem. 2019. PMID: 31535859 Free PMC article.
-
5-Aryl-4-carboxamide-1,3-oxazoles: potent and selective GSK-3 inhibitors.Bioorg Med Chem Lett. 2012 Mar 1;22(5):1989-94. doi: 10.1016/j.bmcl.2012.01.034. Epub 2012 Jan 21. Bioorg Med Chem Lett. 2012. PMID: 22310227
-
Synthesis and evaluation of 8-amino-[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one derivatives as glycogen synthase kinase-3 (GSK-3) inhibitors.Bioorg Med Chem Lett. 2013 Jul 1;23(13):3983-7. doi: 10.1016/j.bmcl.2013.03.119. Epub 2013 Apr 4. Bioorg Med Chem Lett. 2013. PMID: 23683591
-
Natural and synthetic bioactive inhibitors of glycogen synthase kinase.Eur J Med Chem. 2017 Jan 5;125:464-477. doi: 10.1016/j.ejmech.2016.09.058. Epub 2016 Sep 20. Eur J Med Chem. 2017. PMID: 27689729 Review.
-
Targeting Tau Hyperphosphorylation via Kinase Inhibition: Strategy to Address Alzheimer's Disease.Curr Top Med Chem. 2020;20(12):1059-1073. doi: 10.2174/1568026620666200106125910. Curr Top Med Chem. 2020. PMID: 31903881 Review.
Cited by
-
Molecular docking based screening analysis of GSK3B.Bioinformation. 2019 Mar 15;15(3):201-208. doi: 10.6026/97320630015201. eCollection 2019. Bioinformation. 2019. PMID: 31354196 Free PMC article.
-
Biomarker-guided drug therapy: personalized medicine for treating Alzheimer's disease.Neural Regen Res. 2021 Oct;16(10):2010-2011. doi: 10.4103/1673-5374.308079. Neural Regen Res. 2021. PMID: 33642382 Free PMC article. No abstract available.
-
Structural Basis for Achieving GSK-3β Inhibition with High Potency, Selectivity, and Brain Exposure for Positron Emission Tomography Imaging and Drug Discovery.J Med Chem. 2019 Nov 14;62(21):9600-9617. doi: 10.1021/acs.jmedchem.9b01030. Epub 2019 Oct 21. J Med Chem. 2019. PMID: 31535859 Free PMC article.
-
Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward.Front Mol Neurosci. 2022 Jan 21;14:792364. doi: 10.3389/fnmol.2021.792364. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35126052 Free PMC article. Review.
-
Development of [18F]Maleimide-Based Glycogen Synthase Kinase-3β Ligands for Positron Emission Tomography Imaging.ACS Med Chem Lett. 2017 Feb 2;8(3):287-292. doi: 10.1021/acsmedchemlett.6b00405. eCollection 2017 Mar 9. ACS Med Chem Lett. 2017. PMID: 28337318 Free PMC article.
References
-
- Woodgett JR. EMBO J. 1990;9:2431–2438. - PMC - PubMed
- Woodgett JR. Glycogen Synthase Kinase 3 (GSK-3) and Its Inhibitors: Drug Discovery and Development. John Wiley and Sons, Inc.; Hoboken, New Jersey: 2006. pp. 3–23.
- Jope RS, Johnson GVW. Trends Biochem Sci. 2004;29:95–102. - PubMed
- Meijer L, Flajolet M, Greengard P. Trends Pharmacol Sci. 2004;25:471–480. - PubMed
- Lei P, Ayton S, Bush AI, Adlard PA. Int J Alzheimers Dis. 2011;2011:9. - PMC - PubMed
- Thorne CA, Wichaidit C, Coster AD, Posner BA, Wu LF, Altschuler SJ. Nat Chem Biol. 2015;11:58–63. - PMC - PubMed
-
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. EMBO J. 2001;20:27–39. - PMC - PubMed
- Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ. J Neurochem. 2002;83:1529–1533. - PubMed
- Fuster-Matanzo A, Llorens-Martín M, de Barreda EG, Ávila J, Hernández F. PLoS ONE. 2011;6:e27262. - PMC - PubMed
- Sirerol-Piquer M, Gomez-Ramos P, Hernández F, Perez M, Morán MA, Fuster-Matanzo A, Lucas JJ, Avila J, García-Verdugo JM. Hippocampus. 2011;21:910–922. - PubMed
- Leroy K, Yilmaz Z, Brion JP. Neuropathol Appl Neurobiol. 2007;33:43–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

