Protective Effect of Irisin on Atherosclerosis via Suppressing Oxidized Low Density Lipoprotein Induced Vascular Inflammation and Endothelial Dysfunction
- PMID: 27355581
- PMCID: PMC4927070
- DOI: 10.1371/journal.pone.0158038
Protective Effect of Irisin on Atherosclerosis via Suppressing Oxidized Low Density Lipoprotein Induced Vascular Inflammation and Endothelial Dysfunction
Abstract
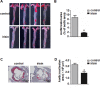
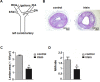
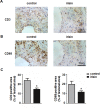
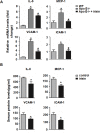
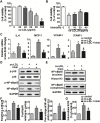
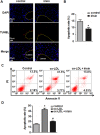
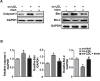
Irisin, a newly discovered myokine, is considered as a promising candidate for the treatment of metabolic disturbances and cardiovascular diseases. In the present study, we used two animal models, apolipoprotein E-deficient mice fed on a high-cholesterol diet and a mouse carotid partial ligation model to test the anti-atherosclerotic effect of irisin. Irisin treatment (0.5 μg/g body weight/day) significantly reduced the severity of aortic atherosclerosis in apolipoprotein E-deficient mice fed on a high-cholesterol diet and suppressed carotid neointima formation in a carotid partial ligation model. It was associated with decreased inflammation and cell apoptosis in aortic tissues. In addition, in a cell culture model, irisin restored ox-LDL-induced human umbilical vein endothelial cell dysfunction by reducing the levels of inflammatory genes via inhibiting the reactive oxygen species (ROS)/ p38 MAPK/ NF-κB signaling pathway activation and inhibiting cell apoptosis via up-regulating Bcl-2 and down-regulating Bax and caspase-3 expression. Our study demonstrated that irisin significantly reduced atherosclerosis in apolipoprotein E-deficient mice via suppressing ox-LDL-induced cell inflammation and apoptosis, which might have a direct therapeutic effect on atherosclerotic diseases.
Conflict of interest statement
Figures
Similar articles
-
Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice.Atherosclerosis. 2015 Dec;243(2):438-48. doi: 10.1016/j.atherosclerosis.2015.10.020. Epub 2015 Oct 19. Atherosclerosis. 2015. PMID: 26520898
-
Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways.Vascul Pharmacol. 2015 Jul;70:23-34. doi: 10.1016/j.vph.2015.03.002. Epub 2015 Apr 4. Vascul Pharmacol. 2015. PMID: 25849952
-
Longxuetongluo capsule inhibits atherosclerosis progression in high-fat diet-induced ApoE-/- mice by improving endothelial dysfunction.Atherosclerosis. 2016 Dec;255:156-163. doi: 10.1016/j.atherosclerosis.2016.08.022. Epub 2016 Aug 23. Atherosclerosis. 2016. PMID: 27591127
-
Current Insights on the Role of Irisin in Endothelial Dysfunction.Curr Vasc Pharmacol. 2022;20(3):205-220. doi: 10.2174/1570161120666220510120220. Curr Vasc Pharmacol. 2022. PMID: 35538838 Review.
-
Atherosclerotic cardiovascular disease: a review of initiators and protective factors.Inflammopharmacology. 2016 Feb;24(1):1-10. doi: 10.1007/s10787-015-0255-y. Epub 2016 Jan 11. Inflammopharmacology. 2016. PMID: 26750181 Review.
Cited by
-
The calcium binding protein S100β marks hedgehog-responsive resident vascular stem cells within vascular lesions.NPJ Regen Med. 2021 Mar 1;6(1):10. doi: 10.1038/s41536-021-00120-8. NPJ Regen Med. 2021. PMID: 33649337 Free PMC article.
-
Expression of Recombinant Rat Secretable FNDC5 in Pichia Pastoris and Detection of Its Biological Activity.Front Endocrinol (Lausanne). 2022 Mar 7;13:852015. doi: 10.3389/fendo.2022.852015. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35321332 Free PMC article.
-
Cardioprotective Effects of Exercise: The Role of Irisin and Exosome.Curr Vasc Pharmacol. 2024;22(5):316-334. doi: 10.2174/0115701611285736240516101803. Curr Vasc Pharmacol. 2024. PMID: 38808716 Review.
-
Inverse correlation between serum irisin and cardiovascular risk factors among Chinese overweight/obese population.BMC Cardiovasc Disord. 2021 Nov 30;21(1):570. doi: 10.1186/s12872-021-02380-0. BMC Cardiovasc Disord. 2021. PMID: 34847893 Free PMC article.
-
Irisin Regulates Cardiac Responses to Exercise in Health and Diseases: a Narrative Review.J Cardiovasc Transl Res. 2023 Apr;16(2):430-442. doi: 10.1007/s12265-022-10310-4. Epub 2022 Aug 29. J Cardiovasc Transl Res. 2023. PMID: 36036861 Review.
References
-
- Peters SA, den Ruijter HM, Bots ML, Moons KG. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart (British Cardiac Society). 2012;98(3):177–84. Epub 2011/11/19. 10.1136/heartjnl-2011-300747 . - DOI - PubMed
-
- Stefano R, Massimiliano C, Marina C, Rossella M, Chiara P, Alessio L, et al. A score including ADAM17 substrates correlates to recurring cardiovascular event in subjects with atherosclerosis. Atherosclerosis. 2015;239(2):459–64. Epub 2015/02/18. 10.1016/j.atherosclerosis.2015.01.029 . - DOI - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

