Dynamic Regulation of Cell Volume and Extracellular ATP of Human Erythrocytes
- PMID: 27355484
- PMCID: PMC4927150
- DOI: 10.1371/journal.pone.0158305
Dynamic Regulation of Cell Volume and Extracellular ATP of Human Erythrocytes
Abstract
Introduction: The peptide mastoparan 7 (MST7) triggered in human erythrocytes (rbcs) the release of ATP and swelling. Since swelling is a well-known inducer of ATP release, and extracellular (ATPe), interacting with P (purinergic) receptors, can affect cell volume (Vr), we explored the dynamic regulation between Vr and ATPe.
Methods and treatments: We made a quantitative assessment of MST7-dependent kinetics of Vr and of [ATPe], both in the absence and presence of blockers of ATP efflux, swelling and P receptors.
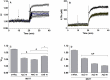
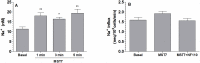
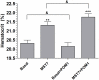
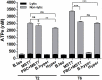
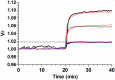
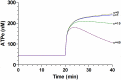
Results: In rbcs 10 μM MST7 promoted acute, strongly correlated changes in [ATPe] and Vr. Whereas MST7 induced increases of 10% in Vr and 190 nM in [ATPe], blocking swelling in a hyperosmotic medium + MST7 reduced [ATPe] by 40%. Pre-incubation of rbcs with 10 μM of either carbenoxolone or probenecid, two inhibitors of the ATP conduit pannexin 1, reduced [ATPe] by 40-50% and swelling by 40-60%, while in the presence of 80 U/mL apyrase, an ATPe scavenger, cell swelling was prevented. While exposure to 10 μM NF110, a blocker of ATP-P2X receptors mediating sodium influx, reduced [ATPe] by 48%, and swelling by 80%, incubation of cells in sodium free medium reduced swelling by 92%.
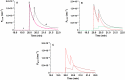
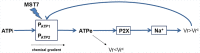
Analysis and discussion: Results were analyzed by means of a mathematical model where ATPe kinetics and Vr kinetics were mutually regulated. Model dependent fit to experimental data showed that, upon MST7 exposure, ATP efflux required a fast 1960-fold increase of ATP permeability, mediated by two kinetically different conduits, both of which were activated by swelling and inactivated by time. Both experimental and theoretical results suggest that, following MST7 exposure, ATP is released via two conduits, one of which is mediated by pannexin 1. The accumulated ATPe activates P2X receptors, followed by sodium influx, resulting in cell swelling, which in turn further activates ATP release. Thus swelling and P2X receptors constitute essential components of a positive feedback loop underlying ATP-induced ATP release of rbcs.
Conflict of interest statement
Figures

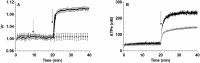


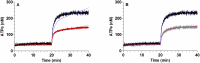
Similar articles
-
Kinetics of extracellular ATP in mastoparan 7-activated human erythrocytes.Biochim Biophys Acta. 2013 Oct;1830(10):4692-707. doi: 10.1016/j.bbagen.2013.05.033. Epub 2013 Jun 4. Biochim Biophys Acta. 2013. PMID: 23742824 Free PMC article.
-
Regulation of extracellular ATP of human erythrocytes treated with α-hemolysin. Effects of cell volume, morphology, rheology and hemolysis.Biochim Biophys Acta Mol Cell Res. 2019 May;1866(5):896-915. doi: 10.1016/j.bbamcr.2019.01.018. Epub 2019 Feb 3. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30726708
-
Regulation of extracellular ATP in human erythrocytes infected with Plasmodium falciparum.PLoS One. 2014 May 23;9(5):e96216. doi: 10.1371/journal.pone.0096216. eCollection 2014. PLoS One. 2014. PMID: 24858837 Free PMC article.
-
Purinergic signaling involved in Müller cell function in the mammalian retina.Prog Retin Eye Res. 2011 Sep;30(5):324-42. doi: 10.1016/j.preteyeres.2011.06.001. Epub 2011 Jun 14. Prog Retin Eye Res. 2011. PMID: 21689780 Review.
-
The participation of plasma membrane hemichannels to purinergic signaling.Biochim Biophys Acta. 2013 Jan;1828(1):79-93. doi: 10.1016/j.bbamem.2012.01.002. Epub 2012 Jan 12. Biochim Biophys Acta. 2013. PMID: 22266266 Review.
Cited by
-
A preliminary study of phosphodiesterases and adenylyl cyclase signaling pathway on red blood cell deformability of sickle cell patients.Front Physiol. 2023 Sep 15;14:1215835. doi: 10.3389/fphys.2023.1215835. eCollection 2023. Front Physiol. 2023. PMID: 37781231 Free PMC article.
-
Interactive Dynamics of Cell Volume and Cell Death in Human Erythrocytes Exposed to α-Hemolysin from Escherichia coli.Int J Mol Sci. 2022 Jan 14;23(2):872. doi: 10.3390/ijms23020872. Int J Mol Sci. 2022. PMID: 35055067 Free PMC article.
-
Induction of ATP Release, PPIX Transport, and Cholesterol Uptake by Human Red Blood Cells Using a New Family of TSPO Ligands.Int J Mol Sci. 2018 Oct 10;19(10):3098. doi: 10.3390/ijms19103098. Int J Mol Sci. 2018. PMID: 30308949 Free PMC article.
-
The Erythrocytic Hypothesis of Brain Energy Crisis in Sporadic Alzheimer Disease: Possible Consequences and Supporting Evidence.J Clin Med. 2020 Jan 12;9(1):206. doi: 10.3390/jcm9010206. J Clin Med. 2020. PMID: 31940879 Free PMC article. Review.
-
Dietary Fatty Acids Affect Red Blood Cell Membrane Composition and Red Blood Cell ATP Release in Dairy Cows.Int J Mol Sci. 2019 Jun 5;20(11):2769. doi: 10.3390/ijms20112769. Int J Mol Sci. 2019. PMID: 31195708 Free PMC article.
References
-
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26: 40–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/1325292 - PubMed
-
- González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91: 1046–55. Available: http://www.ncbi.nlm.nih.gov/pubmed/12456491 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

