Mutations of Vasopressin Receptor 2 Including Novel L312S Have Differential Effects on Trafficking
- PMID: 27355191
- PMCID: PMC4965841
- DOI: 10.1210/me.2016-1002
Mutations of Vasopressin Receptor 2 Including Novel L312S Have Differential Effects on Trafficking
Abstract
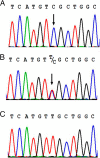
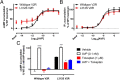
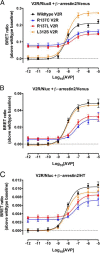
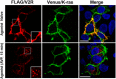
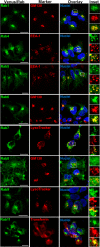
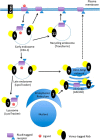
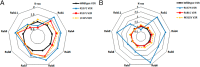
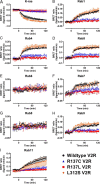
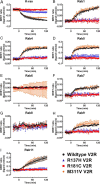
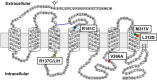
Nephrogenic syndrome of inappropriate antidiuresis (NSIAD) is a genetic disease first described in 2 unrelated male infants with severe symptomatic hyponatremia. Despite undetectable arginine vasopressin levels, patients have inappropriately concentrated urine resulting in hyponatremia, hypoosmolality, and natriuresis. Here, we describe and functionally characterize a novel vasopressin type 2 receptor (V2R) gain-of-function mutation. An L312S substitution in the seventh transmembrane domain was identified in a boy presenting with water-induced hyponatremic seizures at the age of 5.8 years. We show that, compared with wild-type V2R, the L312S mutation results in the constitutive production of cAMP, indicative of the gain-of-function NSIAD profile. Interestingly, like the previously described F229V and I130N NSIAD-causing mutants, this appears to both occur in the absence of notable constitutive β-arrestin2 recruitment and can be reduced by the inverse agonist Tolvaptan. In addition, to understand the effect of various V2R substitutions on the full receptor "life-cycle," we have used and further developed a bioluminescence resonance energy transfer intracellular localization assay using multiple localization markers validated with confocal microscopy. This allowed us to characterize differences in the constitutive and ligand-induced localization and trafficking profiles of the novel L312S mutation as well as for previously described V2R gain-of-function mutants (NSIAD; R137C and R137L), loss-of-function mutants (nephrogenic diabetes insipidus; R137H, R181C, and M311V), and a putative silent V266A V2R polymorphism. In doing so, we describe differences in trafficking between unique V2R substitutions, even at the same amino acid position, therefore highlighting the value of full and thorough characterization of receptor function beyond simple signaling pathway analysis.
Figures
Similar articles
-
Characterization of three vasopressin receptor 2 variants: an apparent polymorphism (V266A) and two loss-of-function mutations (R181C and M311V).PLoS One. 2013 Jun 6;8(6):e65885. doi: 10.1371/journal.pone.0065885. Print 2013. PLoS One. 2013. PMID: 23762448 Free PMC article.
-
Agonist-independent interactions between beta-arrestins and mutant vasopressin type II receptors associated with nephrogenic syndrome of inappropriate antidiuresis.Mol Endocrinol. 2009 Apr;23(4):559-71. doi: 10.1210/me.2008-0321. Epub 2009 Jan 29. Mol Endocrinol. 2009. PMID: 19179480 Free PMC article.
-
Functional characterization of vasopressin type 2 receptor substitutions (R137H/C/L) leading to nephrogenic diabetes insipidus and nephrogenic syndrome of inappropriate antidiuresis: implications for treatments.Mol Pharmacol. 2010 May;77(5):836-45. doi: 10.1124/mol.109.061804. Epub 2010 Feb 16. Mol Pharmacol. 2010. PMID: 20159941 Free PMC article.
-
V2 vasopressin receptor mutations.Vitam Horm. 2020;113:79-99. doi: 10.1016/bs.vh.2019.08.012. Epub 2019 Sep 13. Vitam Horm. 2020. PMID: 32138955 Review.
-
Nephrogenic syndrome of inappropriate antidiuresis (NSIAD): a paradigm for activating mutations causing endocrine dysfunction.Pediatr Endocrinol Rev. 2006 Dec;4 Suppl 1:66-70. Pediatr Endocrinol Rev. 2006. PMID: 17261972 Review.
Cited by
-
Vasopressin receptor 2 mutations in the nephrogenic syndrome of inappropriate antidiuresis show different mechanisms of constitutive activation for G protein coupled receptors.Sci Rep. 2020 Jun 4;10(1):9111. doi: 10.1038/s41598-020-65996-w. Sci Rep. 2020. PMID: 32499611 Free PMC article.
-
GPCR Signaling and Trafficking: The Long and Short of It.Trends Endocrinol Metab. 2017 Mar;28(3):213-226. doi: 10.1016/j.tem.2016.10.007. Epub 2016 Nov 23. Trends Endocrinol Metab. 2017. PMID: 27889227 Free PMC article. Review.
-
Using nanoBRET and CRISPR/Cas9 to monitor proximity to a genome-edited protein in real-time.Sci Rep. 2017 Jun 9;7(1):3187. doi: 10.1038/s41598-017-03486-2. Sci Rep. 2017. PMID: 28600500 Free PMC article.
-
Clinical, Genetic and Functional Characterization of a Novel AVPR2 Missense Mutation in a Woman with X-Linked Recessive Nephrogenic Diabetes Insipidus.J Pers Med. 2022 Jan 17;12(1):118. doi: 10.3390/jpm12010118. J Pers Med. 2022. PMID: 35055433 Free PMC article.
-
Combined Vorinostat and Chloroquine Inhibit Sodium-Iodide Symporter Endocytosis and Enhance Radionuclide Uptake In Vivo.Clin Cancer Res. 2024 Apr 1;30(7):1352-1366. doi: 10.1158/1078-0432.CCR-23-2043. Clin Cancer Res. 2024. PMID: 37921808 Free PMC article.
References
-
- Ball SG. Vasopressin and disorders of water balance: the physiology and pathophysiology of vasopressin. Ann Clin Biochem. 2007;44(pt 5):417–431. - PubMed
-
- Morello JP, Bichet DG. Nephrogenic diabetes insipidus. Annu Rev Physiol. 2001;63:607–630. - PubMed
-
- Birnbaumer M, Seibold A, Gilbert S, et al. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992;357(6376):333–335. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

