The importance of non-HLA antibodies in transplantation
- PMID: 27345243
- PMCID: PMC5669045
- DOI: 10.1038/nrneph.2016.88
The importance of non-HLA antibodies in transplantation
Abstract
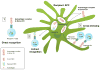
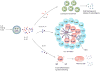
The development of post-transplantation antibodies against non-HLA autoantigens is associated with rejection and decreased long-term graft survival. Although our knowledge of non-HLA antibodies is incomplete, compelling experimental and clinical findings demonstrate that antibodies directed against autoantigens such as angiotensin type 1 receptor, perlecan and collagen, contribute to the process of antibody-mediated acute and chronic rejection. The mechanisms that underlie the production of autoantibodies in the setting of organ transplantation is an important area of ongoing investigation. Ischaemia-reperfusion injury, surgical trauma and/or alloimmune responses can result in the release of organ-derived autoantigens (such as soluble antigens, extracellular vesicles or apoptotic bodies) that are presented to B cells in the context of the transplant recipient's antigen presenting cells and stimulate autoantibody production. Type 17 T helper cells orchestrate autoantibody production by supporting the proliferation and maturation of autoreactive B cells within ectopic tertiary lymphoid tissue. Conversely, autoantibody-mediated graft damage can trigger alloimmunity and the development of donor-specific HLA antibodies that can act in synergy to promote allograft rejection. Identification of the immunologic phenotypes of transplant recipients at risk of non-HLA antibody-mediated rejection, and the development of targeted therapies to treat such rejection, are sorely needed to improve both graft and patient survival.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity.Kidney Int. 2016 Aug;90(2):280-288. doi: 10.1016/j.kint.2016.03.019. Epub 2016 May 14. Kidney Int. 2016. PMID: 27188505 Review.
-
The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes.Transpl Int. 2014 Oct;27(10):1029-38. doi: 10.1111/tri.12371. Epub 2014 Jun 30. Transpl Int. 2014. PMID: 24909812
-
The Emerging Importance of Non-HLA Autoantibodies in Kidney Transplant Complications.J Am Soc Nephrol. 2017 Feb;28(2):400-406. doi: 10.1681/ASN.2016070756. Epub 2016 Oct 17. J Am Soc Nephrol. 2017. PMID: 27798244 Free PMC article. Review.
-
Non-HLA antibodies in solid organ transplantation: recent concepts and clinical relevance.Curr Opin Organ Transplant. 2013 Aug;18(4):430-5. doi: 10.1097/MOT.0b013e3283636e55. Curr Opin Organ Transplant. 2013. PMID: 23838648 Review.
-
Extracellular Vesicles Mediate Immune Responses to Tissue-Associated Self-Antigens: Role in Solid Organ Transplantations.Front Immunol. 2022 Apr 27;13:861583. doi: 10.3389/fimmu.2022.861583. eCollection 2022. Front Immunol. 2022. PMID: 35572510 Free PMC article. Review.
Cited by
-
Liquid biopsies: donor-derived cell-free DNA for the detection of kidney allograft injury.Nat Rev Nephrol. 2021 Sep;17(9):591-603. doi: 10.1038/s41581-021-00428-0. Epub 2021 May 24. Nat Rev Nephrol. 2021. PMID: 34031575 Review.
-
Serological Antibodies against Kidney, Liver, and Spleen Membrane Antigens as Potential Biomarkers in Patients with Immune Disorders.Int J Mol Sci. 2024 Feb 7;25(4):2025. doi: 10.3390/ijms25042025. Int J Mol Sci. 2024. PMID: 38396703 Free PMC article.
-
RORγt inverse agonist TF-S14 inhibits Th17 cytokines and prolongs skin allograft survival in sensitized mice.Commun Biol. 2024 Apr 12;7(1):454. doi: 10.1038/s42003-024-06144-2. Commun Biol. 2024. PMID: 38609465 Free PMC article.
-
Advancing precision in histocompatibility and immunogenetics: a comprehensive review of the UCLA exchange program.Front Genet. 2024 Feb 1;15:1352764. doi: 10.3389/fgene.2024.1352764. eCollection 2024. Front Genet. 2024. PMID: 38362203 Free PMC article.
-
Anti-glutathione S-transferase theta 1 antibodies correlate with graft loss in non-sensitized pediatric kidney recipients.Front Med (Lausanne). 2022 Nov 30;9:1035400. doi: 10.3389/fmed.2022.1035400. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36530923 Free PMC article.
References
-
- Stegall MD, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405–13. - PubMed
-
- Amico P, et al. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87:1681–8. - PubMed
-
- Gloor JM, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582–9. - PubMed
-
- Everly MJ. Incidence and hazards of alloantibodies in renal transplantation. Clin Transpl. 2013:313–7. - PubMed
-
- Wiebe C, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

