Agent-Based Deterministic Modeling of the Bone Marrow Homeostasis
- PMID: 27340402
- PMCID: PMC4910184
- DOI: 10.1155/2016/8054219
Agent-Based Deterministic Modeling of the Bone Marrow Homeostasis
Abstract
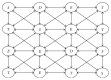
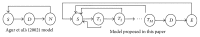
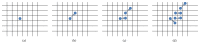
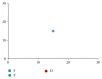
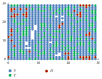
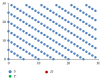
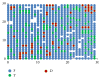
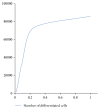
Modeling of stem cells not only describes but also predicts how a stem cell's environment can control its fate. The first stem cell populations discovered were hematopoietic stem cells (HSCs). In this paper, we present a deterministic model of bone marrow (that hosts HSCs) that is consistent with several of the qualitative biological observations. This model incorporates stem cell death (apoptosis) after a certain number of cell divisions and also demonstrates that a single HSC can potentially populate the entire bone marrow. It also demonstrates that there is a production of sufficient number of differentiated cells (RBCs, WBCs, etc.). We prove that our model of bone marrow is biologically consistent and it overcomes the biological feasibility limitations of previously reported models. The major contribution of our model is the flexibility it allows in choosing model parameters which permits several different simulations to be carried out in silico without affecting the homeostatic properties of the model. We have also performed agent-based simulation of the model of bone marrow system proposed in this paper. We have also included parameter details and the results obtained from the simulation. The program of the agent-based simulation of the proposed model is made available on a publicly accessible website.
Figures
Similar articles
-
Mathematical modeling of bone marrow - peripheral blood dynamics in the disease state based on current emerging paradigms, part II.J Theor Biol. 2019 Jan 7;460:37-55. doi: 10.1016/j.jtbi.2018.10.008. Epub 2018 Oct 6. J Theor Biol. 2019. PMID: 30296448
-
Hematopoietic Stem Cell Dynamics Are Regulated by Progenitor Demand: Lessons from a Quantitative Modeling Approach.Stem Cells. 2019 Jul;37(7):948-957. doi: 10.1002/stem.3005. Epub 2019 Apr 3. Stem Cells. 2019. PMID: 30897261
-
Stem cell homeostasis by integral feedback through the niche.J Theor Biol. 2019 Nov 21;481:100-109. doi: 10.1016/j.jtbi.2018.12.029. Epub 2018 Dec 20. J Theor Biol. 2019. PMID: 30579956
-
Current approaches in biomaterial-based hematopoietic stem cell niches.Acta Biomater. 2018 May;72:1-15. doi: 10.1016/j.actbio.2018.03.028. Epub 2018 Mar 22. Acta Biomater. 2018. PMID: 29578087 Review.
-
Mobilization of hematopoietic stem cells: state of the art.Curr Opin Organ Transplant. 2008 Feb;13(1):53-8. doi: 10.1097/MOT.0b013e3282f42473. Curr Opin Organ Transplant. 2008. PMID: 18660707 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

