Role of mTOR Inhibitors in Kidney Disease
- PMID: 27338360
- PMCID: PMC4926507
- DOI: 10.3390/ijms17060975
Role of mTOR Inhibitors in Kidney Disease
Abstract
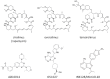
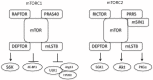
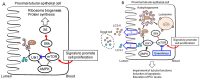
The first compound that inhibited the mammalian target of rapamycin (mTOR), sirolimus (rapamycin) was discovered in the 1970s as a soil bacterium metabolite collected on Easter Island (Rapa Nui). Because sirolimus showed antiproliferative activity, researchers investigated its molecular target and identified the TOR1 and TOR2. The mTOR consists of mTOR complex 1 (mTORC1) and mTORC2. Rapalogues including sirolimus, everolimus, and temsirolimus exert their effect mainly on mTORC1, whereas their inhibitory effect on mTORC2 is mild. To obtain compounds with more potent antiproliferative effects, ATP-competitive inhibitors of mTOR targeting both mTORC1 and mTORC2 have been developed and tested in clinical trials as anticancer drugs. Currently, mTOR inhibitors are used as anticancer drugs against several solid tumors, and immunosuppressive agents for transplantation of various organs. This review discusses the role of mTOR inhibitors in renal disease with a particular focus on renal cancer, diabetic nephropathy, and kidney transplantation.
Keywords: diabetic nephropathy; kidney; kidney transplantation; mTOR inhibitor; renal cancer.
Figures

Similar articles
-
Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes.Adv Biol Regul. 2015 Jan;57:64-74. doi: 10.1016/j.jbior.2014.09.004. Epub 2014 Sep 18. Adv Biol Regul. 2015. PMID: 25442674
-
MLN0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma.Mol Cancer Ther. 2015 Feb;14(2):395-406. doi: 10.1158/1535-7163.MCT-14-0711. Epub 2014 Dec 17. Mol Cancer Ther. 2015. PMID: 25519700 Free PMC article.
-
Potentiation of Growth Inhibitory Responses of the mTOR Inhibitor Everolimus by Dual mTORC1/2 Inhibitors in Cultured Breast Cancer Cell Lines.PLoS One. 2015 Jul 6;10(7):e0131400. doi: 10.1371/journal.pone.0131400. eCollection 2015. PLoS One. 2015. PMID: 26148118 Free PMC article.
-
Cellular and molecular effects of the mTOR inhibitor everolimus.Clin Sci (Lond). 2015 Nov;129(10):895-914. doi: 10.1042/CS20150149. Clin Sci (Lond). 2015. PMID: 26330617 Review.
-
Time now to TORC the TORC? New developments in mTOR pathway inhibition in lymphoid malignancies.Br J Haematol. 2014 Aug;166(3):336-51. doi: 10.1111/bjh.12945. Epub 2014 May 19. Br J Haematol. 2014. PMID: 24842496 Review.
Cited by
-
Elucidating the Influence of MPT-driven necrosis-linked LncRNAs on immunotherapy outcomes, sensitivity to chemotherapy, and mechanisms of cell death in clear cell renal carcinoma.Front Oncol. 2023 Dec 15;13:1276715. doi: 10.3389/fonc.2023.1276715. eCollection 2023. Front Oncol. 2023. PMID: 38162499 Free PMC article.
-
Proteomic Study of Low-Birth-Weight Nephropathy in Rats.Int J Mol Sci. 2021 Sep 24;22(19):10294. doi: 10.3390/ijms221910294. Int J Mol Sci. 2021. PMID: 34638634 Free PMC article.
-
Deregulated MTOR (mechanistic target of rapamycin kinase) is responsible for autophagy defects exacerbating kidney stone development.Autophagy. 2020 Apr;16(4):709-723. doi: 10.1080/15548627.2019.1635382. Epub 2019 Jun 29. Autophagy. 2020. PMID: 31257986 Free PMC article.
-
Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation.Int J Mol Sci. 2023 Jun 18;24(12):10301. doi: 10.3390/ijms241210301. Int J Mol Sci. 2023. PMID: 37373448 Free PMC article. Review.
-
Cellular Senescence and Regulated Cell Death of Tubular Epithelial Cells in Diabetic Kidney Disease.Front Endocrinol (Lausanne). 2022 Jun 28;13:924299. doi: 10.3389/fendo.2022.924299. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35837297 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

