tRNA wobble modifications and protein homeostasis
- PMID: 27335723
- PMCID: PMC4909423
- DOI: 10.1080/21690731.2016.1143076
tRNA wobble modifications and protein homeostasis
Abstract
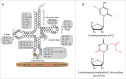
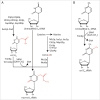
tRNA is a central component of the protein synthesis machinery in the cell. In living cells, tRNAs undergo numerous post-transcriptional modifications. In particular, modifications at the anticodon loop play an important role in ensuring efficient protein synthesis, maintaining protein homeostasis, and helping cell adaptation and survival. Hypo-modification of the wobble position of the tRNA anticodon loop is of particular relevance for translation regulation and is implicated in various human diseases. In this review we summarize recent evidence of how methyl and thiol modifications in eukaryotic tRNA at position 34 affect cellular fitness and modulate regulatory circuits at normal conditions and under stress.
Keywords: HIV-1; decoding; ribosomal frameshifting; ribosome profiling; tRNA modifications; translation regulation.
Figures
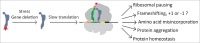
Similar articles
-
tRNA's wobble decoding of the genome: 40 years of modification.J Mol Biol. 2007 Feb 9;366(1):1-13. doi: 10.1016/j.jmb.2006.11.046. Epub 2006 Nov 15. J Mol Biol. 2007. PMID: 17187822 Review.
-
tRNA Modification Profiles and Codon-Decoding Strategies in Methanocaldococcus jannaschii.J Bacteriol. 2019 Apr 9;201(9):e00690-18. doi: 10.1128/JB.00690-18. Print 2019 May 1. J Bacteriol. 2019. PMID: 30745370 Free PMC article.
-
Factors That Shape Eukaryotic tRNAomes: Processing, Modification and Anticodon-Codon Use.Biomolecules. 2017 Mar 8;7(1):26. doi: 10.3390/biom7010026. Biomolecules. 2017. PMID: 28282871 Free PMC article. Review.
-
Beyond the Anticodon: tRNA Core Modifications and Their Impact on Structure, Translation and Stress Adaptation.Genes (Basel). 2024 Mar 19;15(3):374. doi: 10.3390/genes15030374. Genes (Basel). 2024. PMID: 38540433 Free PMC article. Review.
-
Decoding property of C5 uridine modification at the wobble position of tRNA anticodon.Nucleic Acids Res Suppl. 2003;(3):245-6. doi: 10.1093/nass/3.1.245. Nucleic Acids Res Suppl. 2003. PMID: 14510472
Cited by
-
Cytosine-5 RNA methylation links protein synthesis to cell metabolism.PLoS Biol. 2019 Jun 14;17(6):e3000297. doi: 10.1371/journal.pbio.3000297. eCollection 2019 Jun. PLoS Biol. 2019. PMID: 31199786 Free PMC article.
-
Unraveling the RNA modification code with mass spectrometry.Mol Omics. 2020 Aug 1;16(4):305-315. doi: 10.1039/c8mo00247a. Epub 2020 Apr 14. Mol Omics. 2020. PMID: 32285055 Free PMC article.
-
Human TRMT1 catalyzes m2G or m22G formation on tRNAs in a substrate-dependent manner.Sci China Life Sci. 2023 Oct;66(10):2295-2309. doi: 10.1007/s11427-022-2295-0. Epub 2023 May 11. Sci China Life Sci. 2023. PMID: 37204604
-
The thiolation of uridine 34 in tRNA, which controls protein translation, depends on a [4Fe-4S] cluster in the archaeum Methanococcus maripaludis.Sci Rep. 2023 Apr 1;13(1):5351. doi: 10.1038/s41598-023-32423-9. Sci Rep. 2023. PMID: 37005440 Free PMC article.
-
RNA modifications in physiology and disease: towards clinical applications.Nat Rev Genet. 2024 Feb;25(2):104-122. doi: 10.1038/s41576-023-00645-2. Epub 2023 Sep 15. Nat Rev Genet. 2024. PMID: 37714958 Review.
References
-
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 2006; 21:87-96; PMID:16387656; http://dx.doi.org/10.1016/j.molcel.2005.10.036 - DOI - PubMed
-
- Motorin Y, Helm M. tRNA stabilization by modified nucleotides. Biochemistry 2010; 49:4934-44; PMID:20459084; http://dx.doi.org/10.1021/bi100408z - DOI - PubMed
-
- Phizicky EM, Alfonzo JD. Do all modifications benefit all tRNAs? FEBS Lett 2010; 584:265-71; PMID:19931536; http://dx.doi.org/10.1016/j.febslet.2009.11.049 - DOI - PMC - PubMed
-
- Motorin Y, Grosjean H. Multisite-specific tRNA:m5C-methyltransferase (Trm4) in yeast Saccharomyces cerevisiae: identification of the gene and substrate specificity of the enzyme. RNA 1999; 5:1105-18; PMID:10445884; http://dx.doi.org/10.1017/S1355838299982201 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
