Expression of a Mutant kcnj2 Gene Transcript in Zebrafish
- PMID: 27335675
- PMCID: PMC4890933
- DOI: 10.1155/2013/324839
Expression of a Mutant kcnj2 Gene Transcript in Zebrafish
Abstract
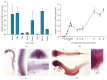
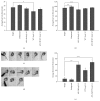
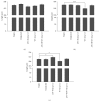
Long QT 7 syndrome (LQT7, also known as Andersen-Tawil syndrome) is a rare autosomal-dominant disorder that causes cardiac arrhythmias, periodic paralysis, and dysmorphic features. Mutations in the human KCNJ2 gene, which encodes for the subunit of the potassium inwardly-rectifying channel (IK1), have been associated with the disorder. The majority of mutations are considered to be dominant-negative as mutant proteins interact to limit the function of wild type KCNJ2 proteins. Several LQT7 syndrome mouse models have been created that vary in the physiological similarity to the human disease. To complement the LQT7 mouse models, we investigated the usefulness of the zebrafish as an alternative model via a transient approach. Initial bioinformatic analysis identified the zebrafish orthologue of the human KCNJ2 gene, together with a spatial expression profile that was similar to that of human. The expression of a kcnj2-12 transcript carrying an in-frame deletion of critical amino acids identified in human studies resulted in embryos that exhibited defects in muscle development, thereby affecting movement, a decrease in jaw size, pupil-pupil distance, and signs of scoliosis. These defects correspond to some phenotypes expressed by human LQT7 patients.
Figures


Similar articles
-
Andersen mutations of KCNJ2 suppress the native inward rectifier current IK1 in a dominant-negative fashion.Cardiovasc Res. 2003 Aug 1;59(2):321-7. doi: 10.1016/s0008-6363(03)00434-6. Cardiovasc Res. 2003. PMID: 12909315
-
Genotype-phenotype correlations of KCNJ2 mutations in Japanese patients with Andersen-Tawil syndrome.Hum Mutat. 2007 Feb;28(2):208. doi: 10.1002/humu.9483. Hum Mutat. 2007. PMID: 17221872
-
Andersen-Tawil syndrome: clinical and molecular aspects.Int J Cardiol. 2013 Dec 5;170(1):1-16. doi: 10.1016/j.ijcard.2013.10.010. Int J Cardiol. 2013. PMID: 24383070 Review.
-
Trafficking-competent and trafficking-defective KCNJ2 mutations in Andersen syndrome.Hum Mutat. 2006 Apr;27(4):388. doi: 10.1002/humu.9418. Hum Mutat. 2006. PMID: 16541386
-
[A new type of periodic paralysis: Andersen-Tawil syndrome].Bull Acad Natl Med. 2008 Nov;192(8):1551-6; discussion 1556-7. Bull Acad Natl Med. 2008. PMID: 19445372 Review. French.
Cited by
-
Inward rectifier potassium current (I K1) and Kir2 composition of the zebrafish (Danio rerio) heart.Pflugers Arch. 2015 Dec;467(12):2437-46. doi: 10.1007/s00424-015-1710-8. Epub 2015 May 21. Pflugers Arch. 2015. PMID: 25991088
-
Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome.J Physiol. 2016 Jun 15;594(12):3245-70. doi: 10.1113/JP271930. Epub 2016 Apr 13. J Physiol. 2016. PMID: 26864374 Free PMC article.
-
Zebrafish heart as a model for human cardiac electrophysiology.Channels (Austin). 2016;10(2):101-10. doi: 10.1080/19336950.2015.1121335. Epub 2015 Dec 15. Channels (Austin). 2016. PMID: 26671745 Free PMC article. Review.
-
Cardiac Ion Channel Regulation in Obesity and the Metabolic Syndrome: Relevance to Long QT Syndrome and Atrial Fibrillation.Front Physiol. 2017 Jun 21;8:431. doi: 10.3389/fphys.2017.00431. eCollection 2017. Front Physiol. 2017. PMID: 28680407 Free PMC article. Review.
-
The power of zebrafish models for understanding the co-occurrence of craniofacial and limb disorders.Genesis. 2021 Feb;59(1-2):e23407. doi: 10.1002/dvg.23407. Epub 2021 Jan 4. Genesis. 2021. PMID: 33393730 Free PMC article. Review.
References
-
- Llobet A., Gasull X., Palés J., Martí E., Gual A. Identification of kir2.1 channel activity in cultured trabecular meshwork cells. Investigative Ophthalmology and Visual Science. 2001;42(10):2371–2379. - PubMed
-
- Zaritsky J. J., Eckman D. M., Wellman G. C., Nelson M. T., Schwarz T. L. Targeted disruption of kir2.1 and kir2.2 genes reveals the essential role of the inwardly rectifying k+ current in k+-mediated vasodilation. Circulation Research. 2000;87(2):160–166. doi: 10.1161/01.RES.87.2.160. - DOI - PubMed
LinkOut - more resources
Full Text Sources
