Human Th17 Cells Lack HIV-Inhibitory RNases and Are Highly Permissive to Productive HIV Infection
- PMID: 27334595
- PMCID: PMC4988157
- DOI: 10.1128/JVI.02869-15
Human Th17 Cells Lack HIV-Inhibitory RNases and Are Highly Permissive to Productive HIV Infection
Abstract
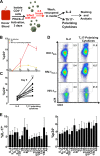
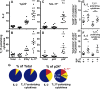
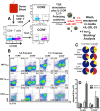
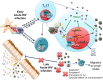
Human immunodeficiency virus (HIV) infects and depletes CD4(+) T cells, but subsets of CD4(+) T cells vary in their susceptibility and permissiveness to infection. For example, HIV preferentially depletes interleukin-17 (IL-17)-producing T helper 17 (Th17) cells and T follicular helper (Tfh) cells. The preferential loss of Th17 cells during the acute phase of infection impairs the integrity of the gut mucosal barrier, which drives chronic immune activation-a key determinant of disease progression. The preferential loss of Th17 cells has been attributed to high CD4, CCR5, and CXCR4 expression. Here, we show that Th17 cells also exhibit heightened permissiveness to productive HIV infection. Primary human CD4(+) T cells were sorted, activated under Th17- or Th0-polarizing conditions and infected, and then analyzed by flow cytometry. Th17-polarizing cytokines increased HIV infection, and HIV infection was disproportionately higher among Th17 cells than among IL-17(-) or gamma interferon-positive (IFN-γ(+)) cells, even upon infection with a replication-defective HIV vector with a pseudotype envelope. Further, Th17-polarized cells produced more viral capsid protein. Our data also reveal that Th17-polarized cells have diminished expression of RNase A superfamily proteins, and we report for the first time that RNase 6 inhibits HIV. Thus, our findings link Th17 polarization to increased HIV replication.
Importance: Our study compares the intracellular replicative capacities of several different HIV isolates among different T cell subsets, providing a link between the differentiation of Th17 cells and HIV replication. Th17 cells are of key importance in mucosal integrity and in the immune response to certain pathogens. Based on our findings and the work of others, we propose a model in which HIV replication is favored by the intracellular environment of two CD4(+) T cell subsets that share several requirements for their differentiation: Th17 and Tfh cells. Characterizing cells that support high levels of viral replication (rather than becoming latently infected or undergoing cell death) informs the search for new therapeutics aimed at manipulating intracellular signaling pathways and/or transcriptional factors that affect HIV replication.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Th17 CD4+ T-Cell as a Preferential Target for HIV Reservoirs.Front Immunol. 2022 Feb 7;13:822576. doi: 10.3389/fimmu.2022.822576. eCollection 2022. Front Immunol. 2022. PMID: 35197986 Free PMC article. Review.
-
Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility.J Immunol. 2011 Dec 1;187(11):6032-42. doi: 10.4049/jimmunol.1101836. Epub 2011 Nov 2. J Immunol. 2011. PMID: 22048765
-
Preferential HIV infection of CCR6+ Th17 cells is associated with higher levels of virus receptor expression and lack of CCR5 ligands.J Virol. 2013 Oct;87(19):10843-54. doi: 10.1128/JVI.01838-13. Epub 2013 Jul 31. J Virol. 2013. PMID: 23903844 Free PMC article.
-
Impaired Th17 polarization of phenotypically naive CD4(+) T-cells during chronic HIV-1 infection and potential restoration with early ART.Retrovirology. 2015 Apr 30;12:38. doi: 10.1186/s12977-015-0164-6. Retrovirology. 2015. PMID: 25924895 Free PMC article.
-
New Th17-specific therapeutic strategies for HIV remission.Curr Opin HIV AIDS. 2019 Mar;14(2):85-92. doi: 10.1097/COH.0000000000000522. Curr Opin HIV AIDS. 2019. PMID: 30543544 Review.
Cited by
-
The transcriptome of HIV-1 infected intestinal CD4+ T cells exposed to enteric bacteria.PLoS Pathog. 2017 Feb 27;13(2):e1006226. doi: 10.1371/journal.ppat.1006226. eCollection 2017 Feb. PLoS Pathog. 2017. PMID: 28241075 Free PMC article.
-
Th17 CD4+ T-Cell as a Preferential Target for HIV Reservoirs.Front Immunol. 2022 Feb 7;13:822576. doi: 10.3389/fimmu.2022.822576. eCollection 2022. Front Immunol. 2022. PMID: 35197986 Free PMC article. Review.
-
A Lachnospiraceae-dominated bacterial signature in the fecal microbiota of HIV-infected individuals from Colombia, South America.Sci Rep. 2018 Mar 14;8(1):4479. doi: 10.1038/s41598-018-22629-7. Sci Rep. 2018. PMID: 29540734 Free PMC article.
-
Functional roles of the human ribonuclease A superfamily in RNA metabolism and membrane receptor biology.Mol Aspects Med. 2019 Dec;70:106-116. doi: 10.1016/j.mam.2019.03.003. Epub 2019 Mar 25. Mol Aspects Med. 2019. PMID: 30902663 Free PMC article. Review.
-
HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood.PLoS One. 2018 Apr 19;13(4):e0192845. doi: 10.1371/journal.pone.0192845. eCollection 2018. PLoS One. 2018. PMID: 29672590 Free PMC article.
References
-
- Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112:2826–2835. doi:10.1182/blood-2008-05-159301. - DOI - PMC - PubMed
-
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14:421–428. doi:10.1038/nm1743. - DOI - PMC - PubMed
-
- Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, Estes JD, Sandler NG, Sukhumvittaya S, Marovich M, Jongrakthaitae S, Akapirat S, Fletscher JL, Kroon E, Dewar R, Trichavaroj R, Chomchey N, Douek DC, O'Connell RJ, Ngauy V, Robb ML, Phanuphak P, Michael NL, Excler JL, Kim JH, de Souza MS, Ananworanich J, RV254/SEARCH 010 and RV304/SEARCH 013 Study Groups. 2014. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 10:e1004543. doi:10.1371/journal.ppat.1004543. - DOI - PMC - PubMed
-
- Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, Zaffiri L, Tryniszewska E, Tsai WP, Vaccari M, Parks RW, Venzon D, Douek DC, O'Shea JJ, Franchini G. 2008. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol 1:279–288. doi:10.1038/mi.2008.14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

