Mode of Ezrin-Membrane Interaction as a Function of PIP2 Binding and Pseudophosphorylation
- PMID: 27332129
- PMCID: PMC4919509
- DOI: 10.1016/j.bpj.2016.05.009
Mode of Ezrin-Membrane Interaction as a Function of PIP2 Binding and Pseudophosphorylation
Abstract
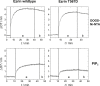
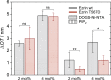
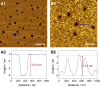
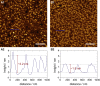
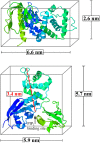
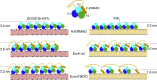
Ezrin, a protein of the ezrin, radixin, moesin (ERM) family, provides a regulated linkage between the plasma membrane and the cytoskeleton. The hallmark of this linkage is the activation of ezrin by phosphatidylinositol-4,5-bisphosphate (PIP2) binding and a threonine phosphorylation at position 567. To analyze the influence of these activating factors on the organization of ezrin on lipid membranes and the proposed concomitant oligomer-monomer transition, we made use of supported lipid bilayers in conjunction with atomic force microscopy and fluorescence microscopy. Bilayers doped with either PIP2 as the natural receptor lipid of ezrin or a Ni-nitrilotriacetic acid-equipped lipid to bind the proteins via their His6-tags to the lipid membrane were used to bind two different ezrin variants: ezrin wild-type and ezrin T567D mimicking the phosphorylated state. Using a combination of reflectometric interference spectroscopy, atomic force microscopy, and Förster resonance energy transfer experiments, we show that only the ezrin T567D mutant, upon binding to PIP2-containing bilayers, undergoes a remarkable conformational change, which we attribute to an opening of the conformation resulting in monomeric protein on the lipid bilayer.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Quantitative analysis of the binding of ezrin to large unilamellar vesicles containing phosphatidylinositol 4,5 bisphosphate.Biophys J. 2008 Feb 1;94(3):1021-33. doi: 10.1529/biophysj.107.110213. Epub 2007 Sep 7. Biophys J. 2008. PMID: 17827228 Free PMC article.
-
Phosphatidylinositol 4,5-bisphosphate alters the number of attachment sites between ezrin and actin filaments: a colloidal probe study.J Biol Chem. 2014 Apr 4;289(14):9833-43. doi: 10.1074/jbc.M113.530659. Epub 2014 Feb 5. J Biol Chem. 2014. PMID: 24500715 Free PMC article.
-
Actin binding of ezrin is activated by specific recognition of PIP2-functionalized lipid bilayers.Biochemistry. 2008 Mar 25;47(12):3762-9. doi: 10.1021/bi702542s. Epub 2008 Feb 27. Biochemistry. 2008. PMID: 18302339
-
Cooperative adsorption of ezrin on PIP2-containing membranes.Biochemistry. 2006 Oct 31;45(43):13025-34. doi: 10.1021/bi061064a. Biochemistry. 2006. PMID: 17059219
-
Activation of F-actin binding capacity of ezrin: synergism of PIP₂ interaction and phosphorylation.Biophys J. 2011 Apr 6;100(7):1708-17. doi: 10.1016/j.bpj.2011.02.039. Biophys J. 2011. PMID: 21463584 Free PMC article.
Cited by
-
Monoallelic loss of the F-actin-binding protein radixin facilitates startle reactivity and pre-pulse inhibition in mice.Front Cell Dev Biol. 2022 Nov 28;10:987691. doi: 10.3389/fcell.2022.987691. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36518539 Free PMC article.
-
Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms.Biochem Biophys Res Commun. 2018 Nov 25;506(2):307-314. doi: 10.1016/j.bbrc.2018.07.155. Epub 2018 Aug 13. Biochem Biophys Res Commun. 2018. PMID: 30139519 Free PMC article. Review.
-
Long-term depression in neurons involves temporal and ultra-structural dynamics of phosphatidylinositol-4,5-bisphosphate relying on PIP5K, PTEN and PLC.Commun Biol. 2023 Apr 3;6(1):366. doi: 10.1038/s42003-023-04726-0. Commun Biol. 2023. PMID: 37012315 Free PMC article.
-
Charge-dependent interactions of monomeric and filamentous actin with lipid bilayers.Proc Natl Acad Sci U S A. 2020 Mar 17;117(11):5861-5872. doi: 10.1073/pnas.1914884117. Epub 2020 Mar 2. Proc Natl Acad Sci U S A. 2020. PMID: 32123101 Free PMC article.
-
A biophysical perspective of the regulatory mechanisms of ezrin/radixin/moesin proteins.Biophys Rev. 2022 Jan 28;14(1):199-208. doi: 10.1007/s12551-021-00928-0. eCollection 2022 Feb. Biophys Rev. 2022. PMID: 35340609 Free PMC article. Review.
References
-
- Bretscher A., Edwards K., Fehon R.G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–599. - PubMed
-
- Mangeat P., Roy C., Martin M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999;9:187–192. - PubMed
-
- Tsukita S., Yonemura S. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J. Biol. Chem. 1999;274:34507–34510. - PubMed
-
- Sauvanet C., Wayt J., Bretscher A. Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells. Annu. Rev. Cell Dev. Biol. 2015;31:593–621. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

