Effect of Nintedanib in Subgroups of Idiopathic Pulmonary Fibrosis by Diagnostic Criteria
- PMID: 27331880
- PMCID: PMC5214917
- DOI: 10.1164/rccm.201602-0402OC
Effect of Nintedanib in Subgroups of Idiopathic Pulmonary Fibrosis by Diagnostic Criteria
Abstract
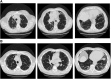
Rationale: In the absence of a surgical lung biopsy, patients diagnosed with idiopathic pulmonary fibrosis (IPF) in clinical practice could participate in the INPULSIS trials of nintedanib if they had honeycombing and/or traction bronchiectasis plus reticulation, without atypical features of usual interstitial pneumonia (UIP), on high-resolution computed tomography (HRCT). Thus, the patients in these trials represented patients with definite UIP and a large subgroup of patients with possible UIP.
Objectives: To investigate the potential impact of diagnostic subgroups on the progression of IPF and the effect of nintedanib.
Methods: We conducted a post hoc subgroup analysis of patients with honeycombing on HRCT and/or confirmation of UIP by biopsy versus patients without either, using pooled data from the INPULSIS trials.
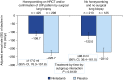
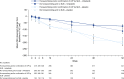
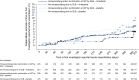
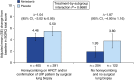
Measurements and main results: Seven hundred twenty-three (68.1%) patients had honeycombing and/or biopsy, and 338 (31.9%) patients had no honeycombing or biopsy. In these subgroups, respectively, the adjusted annual rate of decline in FVC in patients treated with placebo was -225.7 and -221.0 ml/yr, and the nintedanib versus placebo difference in the adjusted annual rate of decline in FVC was 117.0 ml/yr (95% confidence interval, 76.3-157.8) and 98.9 ml/yr (95% confidence interval, 36.4-161.5). There was no significant treatment-by-subgroup interaction (P = 0.8139). Adverse events were similar between the subgroups.
Conclusions: Patients with IPF diagnosed in clinical practice who had possible UIP with traction bronchiectasis on HRCT and had not undergone surgical lung biopsy had disease that progressed in a similar way, and responded similarly to nintedanib, to that of patients with honeycombing on HRCT and/or confirmation of UIP by biopsy.
Keywords: HRCT; diagnosis; high-resolution computed tomography; honeycombing; traction bronchiectasis.
Figures
Comment in
-
What Is in a Pattern? That Which We Call Idiopathic Pulmonary Fibrosis by Any Other Pattern Would Behave Alike!Am J Respir Crit Care Med. 2017 Jan 1;195(1):10-12. doi: 10.1164/rccm.201606-1277ED. Am J Respir Crit Care Med. 2017. PMID: 28035849 Free PMC article. No abstract available.
Similar articles
-
Clinical Course and Changes in High-Resolution Computed Tomography Findings in Patients with Idiopathic Pulmonary Fibrosis without Honeycombing.PLoS One. 2016 Nov 9;11(11):e0166168. doi: 10.1371/journal.pone.0166168. eCollection 2016. PLoS One. 2016. PMID: 27829068 Free PMC article.
-
Usual interstitial pneumonia end-stage features from explants with radiologic and pathological correlations.Ann Diagn Pathol. 2015 Aug;19(4):269-76. doi: 10.1016/j.anndiagpath.2015.05.003. Epub 2015 May 13. Ann Diagn Pathol. 2015. PMID: 26025258
-
Lung function outcomes in the INPULSIS® trials of nintedanib in idiopathic pulmonary fibrosis.Respir Med. 2019 Jan;146:42-48. doi: 10.1016/j.rmed.2018.11.012. Epub 2018 Nov 19. Respir Med. 2019. PMID: 30665517 Clinical Trial.
-
Idiopathic Pulmonary Fibrosis.J Thorac Imaging. 2016 May;31(3):127-39. doi: 10.1097/RTI.0000000000000204. J Thorac Imaging. 2016. PMID: 27043425 Review.
-
Diagnosing fibrotic lung disease: when is high-resolution computed tomography sufficient to make a diagnosis of idiopathic pulmonary fibrosis?Respirology. 2009 Sep;14(7):934-9. doi: 10.1111/j.1440-1843.2009.01626.x. Respirology. 2009. PMID: 19740255 Review.
Cited by
-
Early Diagnosis and Treatment of Idiopathic Pulmonary Fibrosis: A Narrative Review.Pulm Ther. 2023 Jun;9(2):177-193. doi: 10.1007/s41030-023-00216-0. Epub 2023 Feb 11. Pulm Ther. 2023. PMID: 36773130 Free PMC article. Review.
-
Idiopathic Pulmonary Fibrosis (IPF): An Overview.J Clin Med. 2018 Aug 6;7(8):201. doi: 10.3390/jcm7080201. J Clin Med. 2018. PMID: 30082599 Free PMC article. Review.
-
Management of Idiopathic Pulmonary Fibrosis.Ann Pharmacother. 2019 Dec;53(12):1238-1248. doi: 10.1177/1060028019862497. Epub 2019 Jul 7. Ann Pharmacother. 2019. PMID: 31280590 Free PMC article. Review.
-
Idiopathic pulmonary fibrosis: pathogenesis and management.Respir Res. 2018 Feb 22;19(1):32. doi: 10.1186/s12931-018-0730-2. Respir Res. 2018. PMID: 29471816 Free PMC article. Review.
-
Usual interstitial pneumonia: typical, possible, and "inconsistent" patterns.J Bras Pneumol. 2017 Sep-Oct;43(5):393-398. doi: 10.1590/S1806-37562016000000368. J Bras Pneumol. 2017. PMID: 29160385 Free PMC article.
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
-
- Flaherty KR, King TE, Jr, Raghu G, Lynch JP, III, Colby TV, Travis WD, Gross BH, Kazerooni EA, Toews GB, Long Q, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170:904–910. - PubMed
-
- Kreider ME, Hansen-Flaschen J, Ahmad NN, Rossman MD, Kaiser LR, Kucharczuk JC, Shrager JB. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83:1140–1144. - PubMed
-
- Ryerson CJ, Urbania TH, Richeldi L, Mooney JJ, Lee JS, Jones KD, Elicker BM, Koth LL, King TE, Jr, Wolters PJ, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013;42:750–757. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

