Retrospective analysis of the effect of CAPOX and mFOLFOX6 dose intensity on survival in colorectal patients in the adjuvant setting
- PMID: 27330345
- PMCID: PMC4900828
- DOI: 10.3747/co.23.3059
Retrospective analysis of the effect of CAPOX and mFOLFOX6 dose intensity on survival in colorectal patients in the adjuvant setting
Abstract
Background: Despite lack of a true comparative study, the folfox (5-fluorouracil-leucovorin-oxaliplatin) and capox (capecitabine-oxaliplatin) regimens are believed to be similar in their efficacy and tolerability in the treatment of stage iii colorectal cancer. However, that belief has been disputed, because real-life data suggest that the capox regimen is more toxic, leading to more frequent reductions in the delivered dose intensity-thus raising questions about the effect of dose intensity on clinical outcomes.
Methods: A retrospective data review for two Canadian institutions, the Segal Cancer Centre and the Tom Baker Cancer Centre, considered patients diagnosed with stage iii colorectal cancer during 2006-2013. Primary endpoints were dose intensity and toxicity, with a secondary endpoint of disease-free survival.
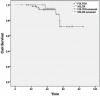
Results: The study enrolled 180 eligible patients (80 at the Segal Cancer Centre, 100 at the Tom Baker Cancer Centre). Of those 180 patients, 75 received capox, and 105 received mfolfox6. In the capox group, a significant dose reduction was identified for capecitabine compared with 5-fluorouracil in mfolfox6 group (p = 0.0014). Similarly, a significant dose reduction was observed for oxaliplatin in mfolfox6 compared with oxaliplatin in capox (p = 0.0001). Compared with the patients receiving capox, those receiving mfolfox6 were twice as likely to experience a treatment delay of more than 1 cycle-length (p = 0.03855). Toxicity was more frequent in patients receiving mfolfox6 (nausea: 30% vs. 18%; diarrhea: 47% vs. 24%; peripheral sensory neuropathy: 32% vs. 3%). At a median follow-up of 40 months, preliminary data showed no difference in disease-free survival (p = 0.598). Pooled data from both institutions were also separately analyzed, and no significant differences were found.
Conclusions: Our results support the use of capox despite a lack of head-to-head randomized trial data.
Keywords: capox; colorectal cancer; disease-free survival; dose intensity; mfolfox6.
Figures
Similar articles
-
Utilization of capecitabine plus oxaliplatin and 5-fluorouracil/folinic acid plus oxaliplatin in the adjuvant treatment of stage IIB and stage III colon cancer: A multi-centre, retrospective, chart review study.J Oncol Pharm Pract. 2018 Oct;24(7):501-506. doi: 10.1177/1078155217718381. Epub 2017 Jul 16. J Oncol Pharm Pract. 2018. PMID: 28714378
-
Real-world tolerance and outcomes of oxaliplatin-based adjuvant chemotherapy for stage III colon cancer-Does dose intensity matter?Asia Pac J Clin Oncol. 2024 Feb;20(1):63-70. doi: 10.1111/ajco.13965. Epub 2023 May 22. Asia Pac J Clin Oncol. 2024. PMID: 37211922
-
Survival Impact of CAPOX Versus FOLFOX in the Adjuvant Treatment of Stage III Colon Cancer.Clin Colorectal Cancer. 2018 Jun;17(2):156-163. doi: 10.1016/j.clcc.2018.01.010. Epub 2018 Feb 7. Clin Colorectal Cancer. 2018. PMID: 29486916
-
Capecitabine: a review.Clin Ther. 2005 Jan;27(1):23-44. doi: 10.1016/j.clinthera.2005.01.005. Clin Ther. 2005. PMID: 15763604 Review.
-
Capecitabine/oxaliplatin as first-line treatment for metastatic colorectal cancer: a meta-analysis.Colorectal Dis. 2010 Jul;12(7):615-23. doi: 10.1111/j.1463-1318.2009.01879.x. Epub 2009 Apr 10. Colorectal Dis. 2010. PMID: 19486086 Review.
Cited by
-
A randomized controlled trial of hand/foot-cooling by hilotherapy to prevent oxaliplatin-related peripheral neuropathy in patients with malignancies of the digestive system.ESMO Open. 2023 Apr;8(2):101205. doi: 10.1016/j.esmoop.2023.101205. Epub 2023 Apr 3. ESMO Open. 2023. PMID: 37018872 Free PMC article. Clinical Trial.
-
Retrospective comparison of efficacy and safety of CAPOX and FOLFOX regimens as adjuvant treatment in patients with stage III colon cancer.J Int Med Res. 2019 Jun;47(6):2507-2515. doi: 10.1177/0300060519848258. Epub 2019 May 17. J Int Med Res. 2019. PMID: 31099282 Free PMC article.
-
Effects of oxaliplatin-containing adjuvant chemotherapy on short-term survival of patients with colon cancer in Dr. Sardjito Hospital, Yogyakarta, Indonesia.J Gastrointest Oncol. 2019 Apr;10(2):226-234. doi: 10.21037/jgo.2018.12.10. J Gastrointest Oncol. 2019. PMID: 31032089 Free PMC article.
References
-
- Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage iii colon cancer: final results from the x-act trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol. 2012;23:1190–7. doi: 10.1093/annonc/mdr366. - DOI - PubMed
-
- Di Costanzo F, Ravasio R, Sobrero A, et al. Capecitabine versus bolus fluorouracil plus leucovorin (folinic acid) as adjuvant chemotherapy for patients with Dukes’ C colon cancer: economic evaluation in an Italian nhs setting. Clin Drug Investig. 2008;28:645–55. doi: 10.2165/00044011-200828100-00005. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources