Dioscin strengthens the efficiency of adriamycin in MCF-7 and MCF-7/ADR cells through autophagy induction: More than just down-regulation of MDR1
- PMID: 27329817
- PMCID: PMC4916600
- DOI: 10.1038/srep28403
Dioscin strengthens the efficiency of adriamycin in MCF-7 and MCF-7/ADR cells through autophagy induction: More than just down-regulation of MDR1
Abstract
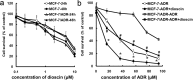
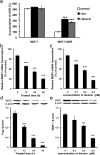
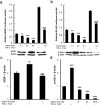
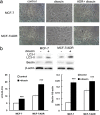
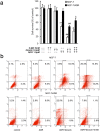
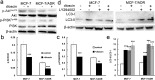
The purpose of present study was to investigate the effect of dioscin on activity of adriamycin (ADR) in ADR-sensitive (MCF-7) and ADR-resistant (MCF-7/ADR) human breast cancer cells and to clarify the molecular mechanisms involved. Antiproliferation effect of ADR was enhanced by dioscin in MCF-7 and MCF-7/ADR cells. Dioscin significantly inhibited MDR1 mRNA and protein expression and MDR1 promoter and nuclear factor κ-B (NF-κB) activity in MCF-7/ADR cells. Additionally, inhibitor κB-α (IκB-α) degradation was inhibited by dioscin. Moreover, dioscin induced the formation of vacuoles in the cytoplasm and protein level of LC3-II in MCF-7 and MCF-7/ADR cells. Autophagy inhibitor 3-MA abolished the effect of dioscin on ADR cytotoxicity. Dioscin inhibited phosphorylation of PI3K and Akt, resulting in upregulation of LC3-II expression. In conclusion, dioscin increased ADR chemosensitivity by down-regulating MDR1 expression through NF-κB signaling inhibition in MCF-7/ADR cells. Autophagy was induced by dioscin to ameliorate the cytotoxicity of ADR via inhibition of the PI3K/AKT pathways in MCF-7 and MCF-7/ADR cells. These findings provide evidence in support of further investigation into the clinical application of dioscin as a chemotherapy adjuvant.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Dioscin restores the activity of the anticancer agent adriamycin in multidrug-resistant human leukemia K562/adriamycin cells by down-regulating MDR1 via a mechanism involving NF-κB signaling inhibition.J Nat Prod. 2013 May 24;76(5):909-14. doi: 10.1021/np400071c. Epub 2013 Apr 26. J Nat Prod. 2013. PMID: 23621869
-
Molecular mechanism of suppression of MDR1 by puerarin from Pueraria lobata via NF-kappaB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP-activated protein kinase in breast cancer MCF-7/adr cells.Mol Nutr Food Res. 2010 Jul;54(7):918-28. doi: 10.1002/mnfr.200900146. Mol Nutr Food Res. 2010. PMID: 20077420
-
Key role of nuclear factor-κB in the cellular pharmacokinetics of adriamycin in MCF-7/Adr cells: the potential mechanism for synergy with 20(S)-ginsenoside Rh2.Drug Metab Dispos. 2012 Oct;40(10):1900-8. doi: 10.1124/dmd.112.045187. Epub 2012 Jun 27. Drug Metab Dispos. 2012. PMID: 22745335
-
Dioscin and diosgenin: Insights into their potential protective effects in cardiac diseases.J Ethnopharmacol. 2021 Jun 28;274:114018. doi: 10.1016/j.jep.2021.114018. Epub 2021 Mar 11. J Ethnopharmacol. 2021. PMID: 33716083 Review.
-
Diosgenin, a steroidal saponin, and its analogs: Effective therapies against different chronic diseases.Life Sci. 2020 Nov 1;260:118182. doi: 10.1016/j.lfs.2020.118182. Epub 2020 Aug 8. Life Sci. 2020. PMID: 32781063 Review.
Cited by
-
Diosgenin and GSK126 Produce Synergistic Effects on Epithelial-Mesenchymal Transition in Gastric Cancer Cells by Mediating EZH2 via the Rho/ROCK Signaling Pathway.Onco Targets Ther. 2020 Jun 8;13:5057-5067. doi: 10.2147/OTT.S237474. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606728 Free PMC article.
-
Recent Advances in the Pharmacological Activities of Dioscin.Biomed Res Int. 2019 Aug 14;2019:5763602. doi: 10.1155/2019/5763602. eCollection 2019. Biomed Res Int. 2019. PMID: 31511824 Free PMC article. Review.
-
Ferroptosis-modulating small molecules for targeting drug-resistant cancer: Challenges and opportunities in manipulating redox signaling.Med Res Rev. 2023 May;43(3):614-682. doi: 10.1002/med.21933. Epub 2023 Jan 19. Med Res Rev. 2023. PMID: 36658724 Free PMC article. Review.
-
Dioscin elicits anti-tumour immunity by inhibiting macrophage M2 polarization via JNK and STAT3 pathways in lung cancer.J Cell Mol Med. 2020 Aug;24(16):9217-9230. doi: 10.1111/jcmm.15563. Epub 2020 Jul 2. J Cell Mol Med. 2020. PMID: 32618105 Free PMC article.
-
Mechanism of ferroptosis in breast cancer and research progress of natural compounds regulating ferroptosis.J Cell Mol Med. 2024 Jan;28(1):e18044. doi: 10.1111/jcmm.18044. Epub 2023 Dec 22. J Cell Mol Med. 2024. PMID: 38140764 Free PMC article. Review.
References
-
- Clarke R. et al.. Molecular and pharmacological aspects of antiestrogen resistance. J. Steroid Biochem. Mol. Biol. 76, 71–84 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

