CD4 T cell epitope specificity determines follicular versus non-follicular helper differentiation in the polyclonal response to influenza infection or vaccination
- PMID: 27329272
- PMCID: PMC4916409
- DOI: 10.1038/srep28287
CD4 T cell epitope specificity determines follicular versus non-follicular helper differentiation in the polyclonal response to influenza infection or vaccination
Abstract
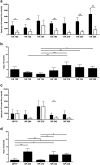
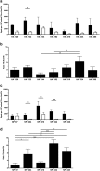
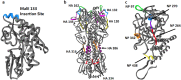
Follicular helper T cells (Tfh) are essential for B cell production of high-affinity, class-switched antibodies. Much interest in Tfh development focuses on the priming environment of CD4 T cells. Here we explored the role that peptide specificity plays in the partitioning of the polyclonal CD4 T cell repertoire between Tfh and NonTfh lineages during the response to influenza. Surprisingly, we found that CD4 T cells specific for different epitopes exhibited distinct tendencies to segregate into Tfh or NonTfh. To alter the microenvironment and abundance, viral antigens were introduced as purified recombinant proteins in adjuvant as native proteins. Also, the most prototypical epitopes were expressed in a completely foreign protein. In many cases, the epitope-specific response patterns of Tfh vs. NonTfh persisted. The functional TcR avidity of only a subset of epitope-specific cells correlated with the tendency to drive a Tfh response. Thus, we conclude that in a polyclonal CD4 T cell repertoire, features of TcR-peptide:MHC class II complex have a strong deterministic influence on the ability of CD4 T cells to become a Tfh or a NonTfh. Our data is most consistent with at least 2 checkpoints of Tfh selection that include both TcR affinity and B cell presentation.
Figures

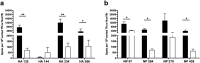
Similar articles
-
Extracellular matrix protein 1 promotes follicular helper T cell differentiation and antibody production.Proc Natl Acad Sci U S A. 2018 Aug 21;115(34):8621-8626. doi: 10.1073/pnas.1801196115. Epub 2018 Aug 7. Proc Natl Acad Sci U S A. 2018. PMID: 30087185 Free PMC article.
-
The peptide specificity of the endogenous T follicular helper cell repertoire generated after protein immunization.PLoS One. 2012;7(10):e46952. doi: 10.1371/journal.pone.0046952. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077537 Free PMC article.
-
Competition within the virus-specific CD4 T-cell pool limits the T follicular helper response after influenza infection.Immunol Cell Biol. 2016 Sep;94(8):729-40. doi: 10.1038/icb.2016.42. Epub 2016 Apr 22. Immunol Cell Biol. 2016. PMID: 27101922
-
Signals that drive T follicular helper cell formation.Immunology. 2017 Oct;152(2):185-194. doi: 10.1111/imm.12778. Epub 2017 Jul 17. Immunology. 2017. PMID: 28628194 Free PMC article. Review.
-
Ex Vivo Culture Assay to Measure Human Follicular Helper T (Tfh) Cell-Mediated Human B Cell Proliferation and Differentiation.Methods Mol Biol. 2018;1707:111-119. doi: 10.1007/978-1-4939-7474-0_8. Methods Mol Biol. 2018. PMID: 29388103 Review.
Cited by
-
Diverse Epitope Specificity, Immunodominance Hierarchy, and Functional Avidity of Effector CD4 T Cells Established During Priming Is Maintained in Lung After Influenza A Virus Infection.Front Immunol. 2018 Apr 6;9:655. doi: 10.3389/fimmu.2018.00655. eCollection 2018. Front Immunol. 2018. PMID: 29681900 Free PMC article.
-
A previously unappreciated polymorphism in the beta chain of I-As expressed in autoimmunity-prone SJL mice: Combined impact on antibody, CD4 T cell recognition and MHC class II dimer structural stability.Mol Immunol. 2022 Mar;143:17-26. doi: 10.1016/j.molimm.2021.12.022. Epub 2022 Jan 5. Mol Immunol. 2022. PMID: 34995990 Free PMC article.
-
Impaired HA-specific T follicular helper cell and antibody responses to influenza vaccination are linked to inflammation in humans.Elife. 2021 Nov 2;10:e70554. doi: 10.7554/eLife.70554. Elife. 2021. PMID: 34726156 Free PMC article.
-
Several Follicular Regulatory T Cell Subsets With Distinct Phenotype and Function Emerge During Germinal Center Reactions.Front Immunol. 2018 Aug 13;9:1792. doi: 10.3389/fimmu.2018.01792. eCollection 2018. Front Immunol. 2018. PMID: 30150980 Free PMC article. Review.
-
Receptor repertoires of murine follicular T helper cells reveal a high clonal overlap in separate lymph nodes in autoimmunity.Elife. 2021 Aug 17;10:e70053. doi: 10.7554/eLife.70053. Elife. 2021. PMID: 34402793 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

