Hepatitis C Core Antigen Testing for Diagnosis of Hepatitis C Virus Infection: A Systematic Review and Meta-analysis
- PMID: 27322622
- PMCID: PMC5345254
- DOI: 10.7326/M16-0065
Hepatitis C Core Antigen Testing for Diagnosis of Hepatitis C Virus Infection: A Systematic Review and Meta-analysis
Abstract
Background: Diagnosis of chronic hepatitis C virus (HCV) infection requires both a positive HCV antibody screen and confirmatory nucleic acid testing (NAT). Testing for hepatitis C virus core antigen (HCVcAg) is a potential alternative to NAT.
Purpose: To evaluate the accuracy of diagnosis of active HCV infection among adults and children for 5 HCVcAg tests compared with NAT.
Data sources: EMBASE, PubMed, Web of Science, Scopus, and Cochrane Database of Systematic Reviews from 1990 through 31 March 2016.
Study selection: Case-control, cross-sectional, cohort, or randomized trials that compared any of 5 HCVcAg tests with an NAT reference standard.
Data extraction: 2 independent reviewers extracted data and assessed quality using an adapted QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies 2) tool.
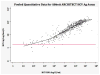
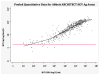
Data synthesis: 44 studies evaluated 5 index tests. Studies for the Abbott ARCHITECT HCV Ag assay had the highest quality, whereas those for the Ortho HCV Ag enzyme-linked immunosorbent assay (ELISA) had the lowest quality. From bivariate analyses, the sensitivity and specificity of the assays were as follows: Abbott ARCHITECT, 93.4% (95% CI, 90.1% to 96.4%) and 98.8% (CI, 97.4% to 99.5%); Ortho ELISA, 93.2% (CI, 81.6% to 97.7%) and 99.2% (CI, 87.9% to 100%); and Hunan Jynda Bioengineering Group HCV Ag ELISA, 59.5% (CI, 46.0% to 71.7%) and 82.9% (CI, 58.6% to 94.3%). Insufficient data were available for a meta-analysis about the Fujirebio Lumipulse Ortho HCV Ag and Eiken Lumispot HCV Ag assays. In 3 quantitative studies using Abbott ARCHITECT, HCVcAg correlated closely with HCV RNA levels greater than 3000 IU/mL.
Limitations: Insufficient data were available on covariates, such as HIV or hepatitis B virus status, for subgroup analyses. Few studies reported genotypes of isolates, and data for genotypes 4, 5, and 6 were scant. Most studies were conducted in high-resource settings and reference laboratories.
Conclusion: The HCVcAg assays with signal amplification have high sensitivity, high specificity, and good correlation with HCV RNA levels greater than 3000 IU/mL and have the potential to replace NAT in settings with high HCV prevalence.
Primary funding source: National Institutes of Health.
Figures


Comment in
-
Newer tests for hepatitis C virus could transform screening.BMJ. 2016 Jun 20;353:i3420. doi: 10.1136/bmj.i3420. BMJ. 2016. PMID: 27330017 No abstract available.
Similar articles
-
Clinical utility of HCV core antigen detection and quantification using serum samples and dried blood spots in people who inject drugs in Dar-es-Salaam, Tanzania.J Int AIDS Soc. 2017 Sep 19;20(1):21856. doi: 10.7448/IAS.20.1.21856. J Int AIDS Soc. 2017. PMID: 28953324 Free PMC article.
-
Diagnostic performance of hepatitis C core antigen assay to identify active infections: A systematic review and meta-analysis.Rev Med Virol. 2023 May;33(3):e2436. doi: 10.1002/rmv.2436. Epub 2023 Feb 22. Rev Med Virol. 2023. PMID: 36811353 Review.
-
Diagnostic Performance of the HCV Core Antigen Test To Identify Hepatitis C in HIV-Infected Patients: a Systematic Review and Meta-Analysis.J Clin Microbiol. 2023 Jan 26;61(1):e0133122. doi: 10.1128/jcm.01331-22. Epub 2022 Dec 20. J Clin Microbiol. 2023. PMID: 36537787 Free PMC article.
-
Hepatitis C virus core antigen: A simplified treatment monitoring tool, including for post-treatment relapse.J Clin Virol. 2017 Jul;92:32-38. doi: 10.1016/j.jcv.2017.05.007. Epub 2017 May 11. J Clin Virol. 2017. PMID: 28521211
-
Hepatitis C core Ag and its clinical applicability: potential advantages and disadvantages for diagnosis and follow-up?Rev Med Virol. 2012 May;22(3):156-65. doi: 10.1002/rmv.717. Epub 2011 Nov 24. Rev Med Virol. 2012. PMID: 22121001 Review.
Cited by
-
Evaluation of Hepatitis C Virus Core Antigen Assay in a Resource-Limited Setting in Pakistan.Diagnostics (Basel). 2021 Jul 28;11(8):1354. doi: 10.3390/diagnostics11081354. Diagnostics (Basel). 2021. PMID: 34441289 Free PMC article.
-
HCV Core-antigen Assay as an Effective Alternative to HCV RNA Quantification in Patients With Hepatitis C.In Vivo. 2023 Jul-Aug;37(4):1498-1503. doi: 10.21873/invivo.13234. In Vivo. 2023. PMID: 37369475 Free PMC article.
-
Virological markers for clinical trials in chronic viral hepatitis.JHEP Rep. 2024 Sep 7;6(11):101214. doi: 10.1016/j.jhepr.2024.101214. eCollection 2024 Nov. JHEP Rep. 2024. PMID: 39524203 Free PMC article. Review.
-
Determining the lower limit of detection required for HCV viral load assay for test of cure following direct-acting antiviral-based treatment regimens: Evidence from a global data set.J Viral Hepat. 2022 Jun;29(6):474-486. doi: 10.1111/jvh.13672. Epub 2022 Mar 30. J Viral Hepat. 2022. PMID: 35278339 Free PMC article.
-
Cost-effectiveness and Budgetary Impact of Hepatitis C Virus Testing, Treatment, and Linkage to Care in US Prisons.Clin Infect Dis. 2020 Mar 17;70(7):1388-1396. doi: 10.1093/cid/ciz383. Clin Infect Dis. 2020. PMID: 31095676 Free PMC article.
References
-
- Gower EEC, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. Journal of Hepatology, Supplement. 2014;61(1 Suppl):S45–57. - PubMed
-
- World Health Organization. Hepatitis C Fact Sheet. 2015.
-
- Ward JW, Mermin JH. Simple, Effective, but Out of Reach? Public Health Implications of HCV Drugs. N Engl J Med. 2015 - PubMed
-
- Kamal SM. Acute hepatitis C: a systematic review. Am J Gastroenterol. 2008;103(5):1283–97. quiz 98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
