The multifaceted role of lysine acetylation in cancer: prognostic biomarker and therapeutic target
- PMID: 27322556
- PMCID: PMC5342454
- DOI: 10.18632/oncotarget.10048
The multifaceted role of lysine acetylation in cancer: prognostic biomarker and therapeutic target
Abstract
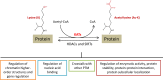
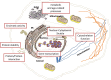
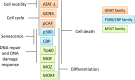
Lysine acetylation is a post-translational modification that regulates gene transcription by targeting histones as well as a variety of transcription factors in the nucleus. Recently, several reports have demonstrated that numerous cytosolic proteins are also acetylated and that this modification, affecting protein activity, localization and stability has profound consequences on their cellular functions. Interestingly, most non-histone proteins targeted by acetylation are relevant for tumorigenesis. In this review, we will analyze the functional implications of lysine acetylation in different cellular compartments, and will examine our current understanding of lysine acetyltransferases family, highlighting the biological role and prognostic value of these enzymes and their substrates in cancer. The latter part of the article will address challenges and current status of molecules targeting lysine acetyltransferase enzymes in cancer therapy.
Keywords: KAT inhibitors; cancer; lysine acetylation; lysine acetyltransferases.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Aberrant lysine acetylation in tumorigenesis: Implications in the development of therapeutics.Pharmacol Ther. 2016 Jun;162:98-119. doi: 10.1016/j.pharmthera.2016.01.011. Epub 2016 Jan 22. Pharmacol Ther. 2016. PMID: 26808162 Review.
-
Chemical Biology Approaches for Investigating the Functions of Lysine Acetyltransferases.Angew Chem Int Ed Engl. 2018 Jan 26;57(5):1162-1184. doi: 10.1002/anie.201704745. Epub 2017 Dec 27. Angew Chem Int Ed Engl. 2018. PMID: 28786225 Review.
-
Genome-scale analysis of regulatory protein acetylation enzymes from photosynthetic eukaryotes.BMC Genomics. 2017 Jul 5;18(1):514. doi: 10.1186/s12864-017-3894-0. BMC Genomics. 2017. PMID: 28679357 Free PMC article.
-
Defining the orphan functions of lysine acetyltransferases.ACS Chem Biol. 2015 Jan 16;10(1):85-94. doi: 10.1021/cb500853p. ACS Chem Biol. 2015. PMID: 25591746 Free PMC article. Review.
-
Measurement and Analysis of Lysine Acetylation by KAT Complexes In Vitro and In Vivo.Methods Mol Biol. 2019;1983:57-77. doi: 10.1007/978-1-4939-9434-2_5. Methods Mol Biol. 2019. PMID: 31087293
Cited by
-
The Role of DNA/Histone Modifying Enzymes and Chromatin Remodeling Complexes in Testicular Germ Cell Tumors.Cancers (Basel). 2018 Dec 20;11(1):6. doi: 10.3390/cancers11010006. Cancers (Basel). 2018. PMID: 30577487 Free PMC article. Review.
-
MS-275 (Entinostat) Promotes Radio-Sensitivity in PAX3-FOXO1 Rhabdomyosarcoma Cells.Int J Mol Sci. 2021 Oct 1;22(19):10671. doi: 10.3390/ijms221910671. Int J Mol Sci. 2021. PMID: 34639012 Free PMC article.
-
Protein Acetylation at the Interface of Genetics, Epigenetics and Environment in Cancer.Metabolites. 2021 Apr 1;11(4):216. doi: 10.3390/metabo11040216. Metabolites. 2021. PMID: 33916219 Free PMC article. Review.
-
Molecular Characterization and Clinical Relevance of Lysine Acetylation Regulators in Urological Cancers.Front Oncol. 2021 May 31;11:647221. doi: 10.3389/fonc.2021.647221. eCollection 2021. Front Oncol. 2021. PMID: 34136387 Free PMC article.
-
SIRT3 regulates cancer cell proliferation through deacetylation of PYCR1 in proline metabolism.Neoplasia. 2019 Jul;21(7):665-675. doi: 10.1016/j.neo.2019.04.008. Epub 2019 May 17. Neoplasia. 2019. PMID: 31108370 Free PMC article.
References
-
- Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annual Review of Biochemistry. 2001;70:81–120. - PubMed
-
- Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn't fit all. Nature reviews. Molecular cell biology. 2007;8:284–95. - PubMed
-
- Kalkhoven E. CBP and p300: HATs for different occasions. Biochemical pharmacology. 2004;68:1145–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

