A recombinant chimeric protein specifically induces mutant KRAS degradation and potently inhibits pancreatic tumor growth
- PMID: 27322423
- PMCID: PMC5190097
- DOI: 10.18632/oncotarget.9996
A recombinant chimeric protein specifically induces mutant KRAS degradation and potently inhibits pancreatic tumor growth
Abstract
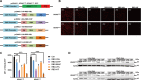
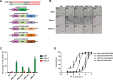
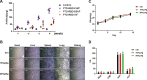
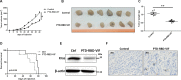
Pancreatic cancer is one of the most lethal human diseases, with an all-stage 5-year survival rate below 5%. To date, no effective and specific therapy is available for this disease. Mutations in KRAS are frequently reported in pancreatic and many other cancers; thus, KRAS is an attractive therapeutic target. Our objective was to specifically eliminate mutant KRAS and induce cell death of tumors expressing this mutant protein. We thus constructed several chimeric proteins by connecting the C-terminal domains of several adaptor proteins of E3 ubiquitin ligases such as CBL, CHIP, E6AP, and VHL, as well as VIF encoded by human immunodeficiency virus type 1 (HIV-1), to the Ras binding domain (RBD) of Raf. Although all of these chimeric proteins caused the degradation of mutant KRAS and the death of KRAS-mutant-tumor cell lines, the RBD-VIF with a protein transduction domain (PTD), named PTD-RBD-VIF, had the strongest tumor-killing effect. Intraperitoneally administered recombinant PTD-RBD-VIF potently inhibited the growth of xenografted KRAS-mutant pancreatic cancer cells. Our findings indicate that recombinant PTD-RBD-VIF, a chimeric protein with a combined cellular-viral origin, could be further developed for the treatment of various tumors harboring mutant or over-activated KRAS, especially for cases presenting with pancreatic cancer recurrence after surgery.
Keywords: KRAS; Ras binding domain; Vif; pancreatic cancer; ubiquitin.
Conflict of interest statement
The authors have declared that no conflicts of interest exists.
Figures


Similar articles
-
Targeted degradation of KRAS by an engineered ubiquitin ligase suppresses pancreatic cancer cell growth in vitro and in vivo.Mol Cancer Ther. 2013 Mar;12(3):286-94. doi: 10.1158/1535-7163.MCT-12-0650. Epub 2013 Jan 3. Mol Cancer Ther. 2013. PMID: 23288781
-
A combinatorial strategy using YAP and pan-RAF inhibitors for treating KRAS-mutant pancreatic cancer.Cancer Lett. 2017 Aug 28;402:61-70. doi: 10.1016/j.canlet.2017.05.015. Epub 2017 May 30. Cancer Lett. 2017. PMID: 28576749
-
Dual Farnesyl and Geranylgeranyl Transferase Inhibitor Thwarts Mutant KRAS-Driven Patient-Derived Pancreatic Tumors.Clin Cancer Res. 2019 Oct 1;25(19):5984-5996. doi: 10.1158/1078-0432.CCR-18-3399. Epub 2019 Jun 21. Clin Cancer Res. 2019. PMID: 31227505 Free PMC article.
-
Critical role of oncogenic KRAS in pancreatic cancer (Review).Mol Med Rep. 2016 Jun;13(6):4943-9. doi: 10.3892/mmr.2016.5196. Epub 2016 Apr 27. Mol Med Rep. 2016. PMID: 27121414 Review.
-
Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: Hopes and realities.Eur J Cancer. 2016 Feb;54:75-83. doi: 10.1016/j.ejca.2015.11.012. Epub 2015 Dec 28. Eur J Cancer. 2016. PMID: 26735353 Review.
Cited by
-
Targeted protein degradation in cancers: Orthodox PROTACs and beyond.Innovation (Camb). 2023 Mar 15;4(3):100413. doi: 10.1016/j.xinn.2023.100413. eCollection 2023 May 15. Innovation (Camb). 2023. PMID: 37033156 Free PMC article. Review.
-
Targeted Intracellular Delivery of Antibodies: The State of the Art.Front Pharmacol. 2018 Oct 24;9:1208. doi: 10.3389/fphar.2018.01208. eCollection 2018. Front Pharmacol. 2018. PMID: 30405420 Free PMC article. Review.
-
Exquisitely Specific anti-KRAS Biodegraders Inform on the Cellular Prevalence of Nucleotide-Loaded States.ACS Cent Sci. 2021 Feb 24;7(2):274-291. doi: 10.1021/acscentsci.0c01337. Epub 2020 Dec 28. ACS Cent Sci. 2021. PMID: 33655066 Free PMC article.
-
PROTAC targeted protein degraders: the past is prologue.Nat Rev Drug Discov. 2022 Mar;21(3):181-200. doi: 10.1038/s41573-021-00371-6. Epub 2022 Jan 18. Nat Rev Drug Discov. 2022. PMID: 35042991 Free PMC article. Review.
-
Biologics-based degraders - an expanding toolkit for targeted-protein degradation.Curr Opin Biotechnol. 2022 Dec;78:102807. doi: 10.1016/j.copbio.2022.102807. Epub 2022 Sep 27. Curr Opin Biotechnol. 2022. PMID: 36179405 Free PMC article. Review.
References
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364:1817–1825. - PubMed
-
- Hidalgo M. Pancreatic cancer. The New England journal of medicine. 2010;362:1605–1617. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

