Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered HSV-1 Expressing IL-12, in Patients with Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma
- PMID: 27314913
- PMCID: PMC4932657
- DOI: 10.1089/humc.2016.031
Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered HSV-1 Expressing IL-12, in Patients with Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma
Abstract
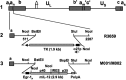
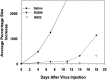
M032 is a second-generation oncolytic herpes simplex virus (oHSV) that selectively replicates in tumor cells. M032 kills tumor cells directly through oncolytic replication and then proceeds to infect tumor cells in proximity, continuing the process of tumor destruction. In addition to this direct oncolytic activity, the virus carries a therapeutic payload-thus acting as a gene therapy vector-and causes the tumor cell to synthesize and secrete the immunity-stimulating protein interleukin-12 (IL-12) before cell death. (1) Human IL-12 is expressed and promotes an immune response against surviving tumor cells, increasing the antitumor effect of the therapy. IL-12 also produces an antiangiogenic effect, by interfering with the production of new tumor blood vessels necessary for tumor growth. Thus, M032 oHSV exerts antitumor effects through three distinct potential mechanisms. The virus has also been genetically engineered to minimize toxic effects for the patient. Preclinical animal models support the safety of intracranial inoculation with M032 in two relevant species (mouse and nonhuman primate). This clinical protocol outlines the dose-escalating phase I study for evaluation of M032 in patients with recurrent or progressive malignant glioma.
Figures

Similar articles
-
Rationale and Design of a Phase 1 Clinical Trial to Evaluate HSV G207 Alone or with a Single Radiation Dose in Children with Progressive or Recurrent Malignant Supratentorial Brain Tumors.Hum Gene Ther Clin Dev. 2017 Mar;28(1):7-16. doi: 10.1089/humc.2017.002. Hum Gene Ther Clin Dev. 2017. PMID: 28319448 Free PMC article. Clinical Trial.
-
Safety and interim survival data after intracranial administration of M032, a genetically engineered oncolytic HSV-1 expressing IL-12, in pet dogs with sporadic gliomas.Neurosurg Focus. 2021 Feb;50(2):E5. doi: 10.3171/2020.11.FOCUS20844. Neurosurg Focus. 2021. PMID: 33524948 Free PMC article.
-
Evaluation of the safety and biodistribution of M032, an attenuated herpes simplex virus type 1 expressing hIL-12, after intracerebral administration to aotus nonhuman primates.Hum Gene Ther Clin Dev. 2014 Mar;25(1):16-27. doi: 10.1089/humc.2013.201. Hum Gene Ther Clin Dev. 2014. PMID: 24649838 Free PMC article.
-
Oncolytic virus therapy using genetically engineered herpes simplex viruses.Front Biosci. 2008 Jan 1;13:2060-4. doi: 10.2741/2823. Front Biosci. 2008. PMID: 17981691 Review.
-
Active immunotherapy: oncolytic virus therapy using HSV-1.Adv Exp Med Biol. 2012;746:178-86. doi: 10.1007/978-1-4614-3146-6_14. Adv Exp Med Biol. 2012. PMID: 22639168 Review.
Cited by
-
Trial Watch: Oncolytic viro-immunotherapy of hematologic and solid tumors.Oncoimmunology. 2018 Aug 27;7(12):e1503032. doi: 10.1080/2162402X.2018.1503032. eCollection 2018. Oncoimmunology. 2018. PMID: 30524901 Free PMC article. Review.
-
Gene Therapy for High Grade Glioma: The Clinical Experience.Expert Opin Biol Ther. 2023 Feb;23(2):145-161. doi: 10.1080/14712598.2022.2157718. Epub 2022 Dec 16. Expert Opin Biol Ther. 2023. PMID: 36510843 Free PMC article. Review.
-
Clinical Applications of Immunotherapy for Recurrent Glioblastoma in Adults.Cancers (Basel). 2023 Jul 31;15(15):3901. doi: 10.3390/cancers15153901. Cancers (Basel). 2023. PMID: 37568717 Free PMC article. Review.
-
Cytokine-armed oncolytic herpes simplex viruses: a game-changer in cancer immunotherapy?J Immunother Cancer. 2024 May 31;12(5):e008025. doi: 10.1136/jitc-2023-008025. J Immunother Cancer. 2024. PMID: 38821716 Free PMC article. Review.
-
Combination Immunotherapy with Vaccine and Oncolytic HSV Virotherapy Is Time Dependent.Mol Cancer Ther. 2024 Sep 4;23(9):1273-1281. doi: 10.1158/1535-7163.MCT-23-0873. Mol Cancer Ther. 2024. PMID: 38710101
References
-
- ClinicalTrials.gov. Genetically Engineered HSV-1 Phase 1 Study (M032-HSV-1). U.S. National Institutes of Health, 2014
-
- Levine A. Principles and Practice of Oncology. (Lippincott Williams and Wilkins, New York: ). 1989
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

