Embelin prevents LMP1-induced TRAIL resistance via inhibition of XIAP in nasopharyngeal carcinoma cells
- PMID: 27313761
- PMCID: PMC4888251
- DOI: 10.3892/ol.2016.4522
Embelin prevents LMP1-induced TRAIL resistance via inhibition of XIAP in nasopharyngeal carcinoma cells
Abstract
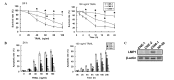
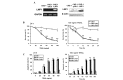
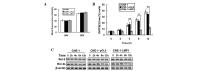
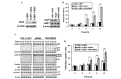
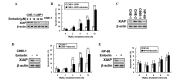
The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) selectively induces apoptosis in the majority of tumor cells, whilst sparing normal cells. However, the potential use of TRAIL in the treatment of cancer is limited by the inevitable emergence of drug resistance. The present study reports the upregulation of latent membrane protein 1 (LMP1)-induced TRAIL resistance via the enhanced expression of X-linked inhibitor of apoptosis protein (XIAP) in nasopharyngeal carcinoma (NPC) cells. LMP1-positive NPC cells were indicated to be more sensitive to TRAIL compared with LMP1-negative NPC cells in three NPC cell lines. CNE-1 is a LMP1-negative NPC cell line that was transfected with pGL6-LMP1; following which, sensitivity to TRAIL decreased. LMP1-induced TRAIL resistance was associated with the decreased cleavage of caspase-8,-3 and -9, BH3 interacting domain death agonist (Bid) and mitochondrial depolarization, without any effects on the expression of the death receptors, B-cell lymphoma (Bcl)-2 and Bcl-extra long. Knockdown of XIAP with small interfering RNA increased caspase-3 and -9 and Bid cleavage, and prevented LMP1-induced TRAIL resistance. Furthermore, embelin, the inhibitor of XIAP, prevented LMP1-induced TRAIL resistance in the Epstein-Barr virus (EBV)-positive CNE-1-LMP1 and C666-1 NPC cell lines. However, embelin did not enhance TRAIL-induced apoptosis in NP-69, which was used as a benign nasopharyngeal epithelial cell line. These data show that LMP1 inhibits TRAIL-mediated apoptosis by upregulation of XIAP. Embelin may be used in an efficacious and safe manner to prevent LMP1-induced TRAIL resistance. The present study may have implications for the development and validation of novel strategies to prevent TRAIL resistance in EBV-positive NPC.
Keywords: X-linked inhibitor of apoptosis protein; apoptosis; embelin; latent membrane protein 1; nasopharyngeal carcinoma; tumor necrosis factor-related apoptosis-inducing ligand.
Figures

Similar articles
-
Latent membrane protein 1 mediates the resistance of nasopharyngeal carcinoma cells to TRAIL-induced apoptosis by activation of the PI3K/Akt signaling pathway.Oncol Rep. 2011 Dec;26(6):1573-9. doi: 10.3892/or.2011.1423. Epub 2011 Aug 17. Oncol Rep. 2011. PMID: 21850380
-
[Inhibition of PI3K-Akt pathway reverses LMP1 induced TRAIL resistance in nasopharyngeal carcinoma cell].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016 May 5;30(9):744-747. doi: 10.13201/j.issn.1001-1781.2016.09.019. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016. PMID: 29771028 Chinese.
-
Simultaneously targeting bcl-2 and Akt pathways sensitizes nasopharyngeal carcinoma to tumor necrosis factor-related apoptosis-inducing ligand.Cancer Biother Radiopharm. 2012 Feb;27(1):88-95. doi: 10.1089/cbr.2011.1031. Epub 2011 Sep 21. Cancer Biother Radiopharm. 2012. PMID: 21936689
-
Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma.Cancer Lett. 2013 Aug 28;337(1):1-7. doi: 10.1016/j.canlet.2013.05.018. Epub 2013 May 17. Cancer Lett. 2013. PMID: 23689138 Review.
-
Embelin and Its Derivatives: Design, Synthesis, and Potential Delivery Systems for Cancer Therapy.Pharmaceuticals (Basel). 2022 Sep 9;15(9):1131. doi: 10.3390/ph15091131. Pharmaceuticals (Basel). 2022. PMID: 36145352 Free PMC article. Review.
Cited by
-
Knockdown of miR-27a sensitizes colorectal cancer stem cells to TRAIL by promoting the formation of Apaf-1-caspase-9 complex.Oncotarget. 2017 Jul 11;8(28):45213-45223. doi: 10.18632/oncotarget.16779. Oncotarget. 2017. PMID: 28423356 Free PMC article.
-
MSC-Delivered Soluble TRAIL and Paclitaxel as Novel Combinatory Treatment for Pancreatic Adenocarcinoma.Theranostics. 2019 Jan 1;9(2):436-448. doi: 10.7150/thno.27576. eCollection 2019. Theranostics. 2019. PMID: 30809285 Free PMC article.
-
Combined application of Embelin and tumor necrosis factor-related apoptosis-inducing ligand inhibits proliferation and invasion in osteosarcoma cells via caspase-induced apoptosis.Oncol Lett. 2018 May;15(5):6931-6940. doi: 10.3892/ol.2018.8209. Epub 2018 Mar 8. Oncol Lett. 2018. PMID: 29731867 Free PMC article.
-
Potential biological process of X-linked inhibitor of apoptosis protein in renal cell carcinoma based upon differential protein expression analysis.Oncol Lett. 2018 Jan;15(1):821-832. doi: 10.3892/ol.2017.7383. Epub 2017 Nov 9. Oncol Lett. 2018. PMID: 29403558 Free PMC article.
-
Targeting of X-linked inhibitor of apoptosis protein and PI3-kinase/AKT signaling by embelin suppresses growth of leukemic cells.PLoS One. 2017 Jul 13;12(7):e0180895. doi: 10.1371/journal.pone.0180895. eCollection 2017. PLoS One. 2017. PMID: 28704451 Free PMC article.
References
-
- Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, Mukhtar H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–2063. doi: 10.1038/sj.onc.1210840. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
