Cinnamide Derivatives of d-Mannose as Inhibitors of the Bacterial Virulence Factor LecB from Pseudomonas aeruginosa
- PMID: 27308201
- PMCID: PMC4906503
- DOI: 10.1002/open.201500162
Cinnamide Derivatives of d-Mannose as Inhibitors of the Bacterial Virulence Factor LecB from Pseudomonas aeruginosa
Abstract
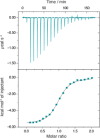
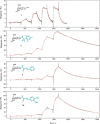
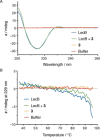
Pseudomonas aeruginosa is an opportunistic Gram-negative pathogen with high antibiotic resistance. Its lectin LecB was identified as a virulence factor and is relevant in bacterial adhesion and biofilm formation. Inhibition of LecB with carbohydrate-based ligands results in a decrease in toxicity and biofilm formation. We recently discovered two classes of potent drug-like glycomimetic inhibitors, that is, sulfonamides and cinnamides of d-mannose. Here, we describe the chemical synthesis and biochemical evaluation of more than 20 derivatives with increased potency compared to the unsubstituted cinnamide. The structure-activity relationship (SAR) obtained and the extended biophysical characterization allowed the experimental determination of the binding mode of these cinnamides with LecB. The established surface binding mode now allows future rational structure-based drug design. Importantly, all glycomimetics tested showed extended receptor residence times with half-lives in the 5-20 min range, a prerequisite for therapeutic application. Thus, the glycomimetics described here provide an excellent basis for future development of anti-infectives against this multidrug-resistant pathogen.
Keywords: LecB/PA-IIL; carbohydrates; glycoconjugates; glycomimetics; lectins.
Figures

Similar articles
-
Discovery of two classes of potent glycomimetic inhibitors of Pseudomonas aeruginosa LecB with distinct binding modes.ACS Chem Biol. 2013 Aug 16;8(8):1775-84. doi: 10.1021/cb400371r. Epub 2013 Jun 28. ACS Chem Biol. 2013. PMID: 23719508
-
Synthesis of mannoheptose derivatives and their evaluation as inhibitors of the lectin LecB from the opportunistic pathogen Pseudomonas aeruginosa.Carbohydr Res. 2015 Aug 14;412:34-42. doi: 10.1016/j.carres.2015.04.010. Epub 2015 May 5. Carbohydr Res. 2015. PMID: 26004349
-
A biophysical study with carbohydrate derivatives explains the molecular basis of monosaccharide selectivity of the Pseudomonas aeruginosa lectin LecB.PLoS One. 2014 Nov 21;9(11):e112822. doi: 10.1371/journal.pone.0112822. eCollection 2014. PLoS One. 2014. PMID: 25415418 Free PMC article.
-
Structural Considerations for Building Synthetic Glycoconjugates as Inhibitors for Pseudomonas aeruginosa Lectins.ChemMedChem. 2022 Jun 20;17(12):e202200081. doi: 10.1002/cmdc.202200081. Epub 2022 May 3. ChemMedChem. 2022. PMID: 35426976 Free PMC article. Review.
-
Structures of the lectins from Pseudomonas aeruginosa: insight into the molecular basis for host glycan recognition.Microbes Infect. 2004 Feb;6(2):221-8. doi: 10.1016/j.micinf.2003.10.016. Microbes Infect. 2004. PMID: 15049333 Review.
Cited by
-
Directing Drugs to Bugs: Antibiotic-Carbohydrate Conjugates Targeting Biofilm-Associated Lectins of Pseudomonas aeruginosa.J Med Chem. 2020 Oct 22;63(20):11707-11724. doi: 10.1021/acs.jmedchem.0c00856. Epub 2020 Oct 2. J Med Chem. 2020. PMID: 32924479 Free PMC article.
-
Anti-biofilm Agents against Pseudomonas aeruginosa: A Structure-Activity Relationship Study of C-Glycosidic LecB Inhibitors.J Med Chem. 2019 Oct 24;62(20):9201-9216. doi: 10.1021/acs.jmedchem.9b01120. Epub 2019 Oct 11. J Med Chem. 2019. PMID: 31553873 Free PMC article.
-
Glycans in drug discovery.Medchemcomm. 2019 Jul 26;10(10):1678-1691. doi: 10.1039/c9md00292h. eCollection 2019 Oct 1. Medchemcomm. 2019. PMID: 31814952 Free PMC article. Review.
-
The assessment of Pseudomonas aeruginosa lectin LecA binding characteristics of divalent galactosides using multiple techniques.Glycobiology. 2021 Dec 18;31(11):1490-1499. doi: 10.1093/glycob/cwab074. Glycobiology. 2021. PMID: 34255029 Free PMC article.
-
Targeting undruggable carbohydrate recognition sites through focused fragment library design.Commun Chem. 2022 May 20;5(1):64. doi: 10.1038/s42004-022-00679-3. Commun Chem. 2022. PMID: 36697615 Free PMC article.
References
-
- Bjarnsholt T., Ciofu O., Molin S., Givskov M., Høiby N., Nat. Rev. Drug Discovery 2013, 12, 791–808. - PubMed
-
- Davies D., Nat. Rev. Drug Discovery 2003, 2, 114–122. - PubMed
-
- Adam E. C., Mitchell B. S., Schumacher D. U., Grant G., Schumacher U., Am. J. Respir. Crit. Care Med. 1997, 155, 2102–2104. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

