Targeting orphan G protein-coupled receptors for the treatment of diabetes and its complications: C-peptide and GPR146
- PMID: 27306986
- PMCID: PMC6092955
- DOI: 10.1111/joim.12528
Targeting orphan G protein-coupled receptors for the treatment of diabetes and its complications: C-peptide and GPR146
Abstract
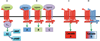
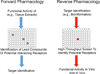
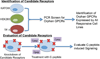
G protein-coupled receptors (GPCRs) are the most abundant receptor family encoded by the human genome and are the targets of a high percentage of drugs currently in use or in clinical trials for the treatment of diseases such as diabetes and its associated complications. Thus, orphan GPCRs, for which the ligand is unknown, represent an important untapped source of therapeutic potential for the treatment of many diseases. We have identified the previously orphan GPCR, GPR146, as the putative receptor of proinsulin C-peptide, which may prove to be an effective treatment for diabetes-associated complications. For example, we have found a potential role of C-peptide and GPR146 in regulating the function of the retinal pigment epithelium, a monolayer of cells in the retina that serves as part of the blood-retinal barrier and is disrupted in diabetic macular oedema. However, C-peptide signalling in this cell type appears to depend at least in part on extracellular glucose concentration and its interaction with insulin. In this review, we discuss the therapeutic potential of orphan GPCRs with a special focus on C-peptide and GPR146, including past and current strategies used to 'deorphanize' this diverse family of receptors, past successes and the inherent difficulties of this process.
Keywords: C-peptide; G protein-coupled receptor; GPR146; diabetes; diabetes-associated complications; orphan GPCR.
© 2016 The Association for the Publication of the Journal of Internal Medicine.
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
Figures
Similar articles
-
Evidence for an interaction between proinsulin C-peptide and GPR146.J Endocrinol. 2013 Jul 11;218(2):B1-8. doi: 10.1530/JOE-13-0203. Print 2013. J Endocrinol. 2013. PMID: 23759446
-
Evidence for an interaction between proinsulin C-peptide and GPR146.J Endocrinol. 2013;218(2):B1-8. J Endocrinol. 2013. PMID: 23980258
-
Is GPR146 really the receptor for proinsulin C-peptide?Bioorg Med Chem Lett. 2020 Jul 1;30(13):127208. doi: 10.1016/j.bmcl.2020.127208. Epub 2020 Apr 20. Bioorg Med Chem Lett. 2020. PMID: 32354568
-
[NEW ACHIEVEMENTS IN THE STUDY OF THE MECHANISMS AND TARGETS OF ACTION OF PROINSULIN C-PEPTIDE].Tsitologiia. 2015;57(6):405-14. Tsitologiia. 2015. PMID: 26495706 Review. Russian.
-
Orphan GPR146: an alternative therapeutic pathway to achieve cholesterol homeostasis?Trends Endocrinol Metab. 2022 Jul;33(7):481-492. doi: 10.1016/j.tem.2022.04.008. Epub 2022 May 10. Trends Endocrinol Metab. 2022. PMID: 35550855 Review.
Cited by
-
A C-peptide complex with albumin and Zn2+ increases measurable GLUT1 levels in membranes of human red blood cells.Sci Rep. 2020 Oct 15;10(1):17493. doi: 10.1038/s41598-020-74527-6. Sci Rep. 2020. PMID: 33060722 Free PMC article.
-
C-Peptide as a Therapy for Type 1 Diabetes Mellitus.Biomedicines. 2021 Mar 8;9(3):270. doi: 10.3390/biomedicines9030270. Biomedicines. 2021. PMID: 33800470 Free PMC article. Review.
-
Regulation of mTORC1 by Upstream Stimuli.Genes (Basel). 2020 Aug 25;11(9):989. doi: 10.3390/genes11090989. Genes (Basel). 2020. PMID: 32854217 Free PMC article. Review.
-
Elimination of GPR146-mediated antiviral function through IRF3/HES1-signalling pathway.Immunology. 2017 Sep;152(1):102-114. doi: 10.1111/imm.12752. Epub 2017 Jun 1. Immunology. 2017. PMID: 28464285 Free PMC article.
-
Novel Human Insulin Isoforms and Cα-Peptide Product in Islets of Langerhans and Choroid Plexus.Diabetes. 2021 Dec;70(12):2947-2956. doi: 10.2337/db21-0198. Epub 2021 Oct 14. Diabetes. 2021. PMID: 34649926 Free PMC article.
References
-
- Collins S, Lohse MJ, O'Dowd B, Caron MG, Lefkowitz RJ. Structure and regulation of G protein-coupled receptors: the beta 2-adrenergic receptor as a model. Vitamins and hormones. 1991;46:1–39. PubMed PMID: 1660639. - PubMed
-
- Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007;190(1):9–19. PubMed PMID: 17428228. - PubMed
-
- Daaka Y, Pitcher JA, Richardson M, Stoffel RH, Robishaw JD, Lefkowitz RJ. Receptor and G betagamma isoform-specific interactions with G protein-coupled receptor kinases. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2180–2185. Epub 1997/03/18. PubMed PMID: 9122168; PubMed Central PMCID: PMCPmc20061. - PMC - PubMed
-
- Jiang Y, Xie X, Zhang Y, Luo X, Wang X, Fan F, et al. Regulation of G-protein signaling by RKTG via sequestration of the G betagamma subunit to the Golgi apparatus. Molecular and cellular biology. 2010;30(1):78–90. Epub 2009/11/04. PubMed PMID: 19884349; PubMed Central PMCID: PMCPmc2798304. - PMC - PubMed
-
- Busnelli M, Sauliere A, Manning M, Bouvier M, Gales C, Chini B. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. The Journal of biological chemistry. 2012;287(6):3617–3629. Epub 2011/11/10. PubMed PMID: 22069312; PubMed Central PMCID: PMCPmc3281696. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

