Differential Regulation of the Two Ferrochelatase Paralogues in Shewanella loihica PV-4 in Response to Environmental Stresses
- PMID: 27287322
- PMCID: PMC4988212
- DOI: 10.1128/AEM.00203-16
Differential Regulation of the Two Ferrochelatase Paralogues in Shewanella loihica PV-4 in Response to Environmental Stresses
Abstract
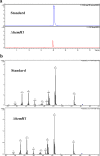
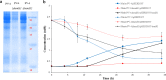
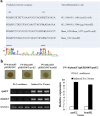
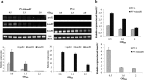
Determining the function and regulation of paralogues is important in understanding microbial functional genomics and environmental adaptation. Heme homeostasis is crucial for the survival of environmental microorganisms. Most Shewanella species encode two paralogues of ferrochelatase, the terminal enzyme in the heme biosynthesis pathway. The function and transcriptional regulation of two ferrochelatase genes, hemH1 and hemH2, were investigated in Shewanella loihica PV-4. The disruption of hemH1 but not hemH2 resulted in a significant accumulation of extracellular protoporphyrin IX (PPIX), the precursor to heme, and decreased intracellular heme levels. hemH1 was constitutively expressed, and the expression of hemH2 increased when hemH1 was disrupted. The transcription of hemH1 was regulated by the housekeeping sigma factor RpoD and potentially regulated by OxyR, while hemH2 appeared to be regulated by the oxidative stress-associated sigma factor RpoE2. When an oxidative stress condition was mimicked by adding H2O2 to the medium or exposing the culture to light, PPIX accumulation was suppressed in the ΔhemH1 mutant. Consistently, transcriptome analysis indicated enhanced iron uptake and suppressed heme synthesis in the ΔhemH1 mutant. These data indicate that the two paralogues are functional in the heme synthesis pathway but regulated by environmental conditions, providing insights into the understanding of bacterial response to environmental stresses and a great potential to commercially produce porphyrin compounds.
Importance: Shewanella is capable of utilizing a variety of electron acceptors for anaerobic respiration because of the existence of multiple c-type cytochromes in which heme is an essential component. The cytochrome-mediated electron transfer across cellular membranes could potentially be used for biotechnological purposes, such as electricity generation in microbial fuel cells and dye decolorization. However, the mechanism underlying the regulation of biosynthesis of heme and cytochromes is poorly understood. Our study has demonstrated that two ferrochelatase genes involved in heme biosynthesis are differentially regulated in response to environmental stresses, including light and reactive oxygen species. This is an excellent example showing how bacteria have evolved to maintain cellular heme homeostasis. More interestingly, the high yields of extracellular protoporphyrin IX by the Shewanella loihica PV-4 mutants could be utilized for commercial production of this valuable chemical via bacterial fermentation.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Differential gene content and gene expression for bacterial evolution and speciation of Shewanella in terms of biosynthesis of heme and heme-requiring proteins.BMC Microbiol. 2019 Jul 30;19(1):173. doi: 10.1186/s12866-019-1549-9. BMC Microbiol. 2019. PMID: 31362704 Free PMC article.
-
An extracytoplasmic function sigma factor-dependent periplasmic glutathione peroxidase is involved in oxidative stress response of Shewanella oneidensis.BMC Microbiol. 2015 Feb 18;15:34. doi: 10.1186/s12866-015-0357-0. BMC Microbiol. 2015. PMID: 25887418 Free PMC article.
-
Regulation of Gene Expression in Shewanella oneidensis MR-1 during Electron Acceptor Limitation and Bacterial Nanowire Formation.Appl Environ Microbiol. 2016 Aug 15;82(17):5428-43. doi: 10.1128/AEM.01615-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27342561 Free PMC article.
-
N-Methyl Protoporphyrin IX: An Understudied Porphyrin.Chem Res Toxicol. 2022 Dec 19;35(12):2186-2193. doi: 10.1021/acs.chemrestox.2c00214. Epub 2022 Dec 2. Chem Res Toxicol. 2022. PMID: 36459538 Free PMC article. Review.
-
Ferrochelatase: Mapping the Intersection of Iron and Porphyrin Metabolism in the Mitochondria.Front Cell Dev Biol. 2022 May 12;10:894591. doi: 10.3389/fcell.2022.894591. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646904 Free PMC article. Review.
Cited by
-
Dissimilatory Nitrate Reduction to Ammonium (DNRA) and Denitrification Pathways Are Leveraged by Cyclic AMP Receptor Protein (CRP) Paralogues Based on Electron Donor/Acceptor Limitation in Shewanella loihica PV-4.Appl Environ Microbiol. 2021 Jan 4;87(2):e01964-20. doi: 10.1128/AEM.01964-20. Print 2021 Jan 4. Appl Environ Microbiol. 2021. PMID: 33158888 Free PMC article.
-
Heme homeostasis and its regulation by hemoproteins in bacteria.mLife. 2024 Jul 11;3(3):327-342. doi: 10.1002/mlf2.12120. eCollection 2024 Sep. mLife. 2024. PMID: 39359680 Free PMC article. Review.
-
Pleiotropic Effects of Hfq on the Cytochrome c Content and Pyomelanin Production in Shewanella oneidensis.Appl Environ Microbiol. 2022 Sep 22;88(18):e0128922. doi: 10.1128/aem.01289-22. Epub 2022 Sep 8. Appl Environ Microbiol. 2022. PMID: 36073941 Free PMC article.
-
Differential gene content and gene expression for bacterial evolution and speciation of Shewanella in terms of biosynthesis of heme and heme-requiring proteins.BMC Microbiol. 2019 Jul 30;19(1):173. doi: 10.1186/s12866-019-1549-9. BMC Microbiol. 2019. PMID: 31362704 Free PMC article.
-
Adaptation to Photooxidative Stress: Common and Special Strategies of the Alphaproteobacteria Rhodobacter sphaeroides and Rhodobacter capsulatus.Microorganisms. 2020 Feb 19;8(2):283. doi: 10.3390/microorganisms8020283. Microorganisms. 2020. PMID: 32093084 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

