Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians
- PMID: 27283835
- PMCID: PMC5129629
- DOI: 10.1007/s10549-016-3847-3
Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians
Abstract
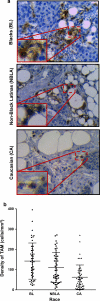
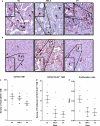
Racial disparities in breast cancer incidence and outcome are a major health care challenge. Patients in the black race group more likely present with an early onset and more aggressive disease. The occurrence of high numbers of macrophages is associated with tumor progression and poor prognosis in solid malignancies. Macrophages are observed in adipose tissues surrounding dead adipocytes in "crown-like structures" (CLS). Here we investigated whether the numbers of CD163+ tumor-associated macrophages (TAMs) and/or CD163+ CLS are associated with patient survival and whether there are significant differences across blacks, non-black Latinas, and Caucasians. Our findings confirm that race is statistically significantly associated with the numbers of TAMs and CLS in breast cancer, and demonstrate that the highest numbers of CD163+ TAM/CLS are found in black breast cancer patients. Our results reveal that the density of CD206 (M2) macrophages is a significant predictor of progression-free survival univariately and is also significant after adjusting for race and for HER2, respectively. We examined whether the high numbers of TAMs detected in tumors from black women were associated with macrophage proliferation, using the Ki-67 nuclear proliferation marker. Our results reveal that TAMs actively divide when in contact with tumor cells. There is a higher ratio of proliferating macrophages in tumors from black patients. These findings suggest that interventions based on targeting TAMs may not only benefit breast cancer patients in general but also serve as an approach to remedy racial disparity resulting in better prognosis patients from minority racial groups.
Keywords: Breast cancer; Crown-like structures; Inflammation; Macrophages; Race/ethnicities.
Figures



Similar articles
-
Macrophages as independent prognostic factors in small T1 breast cancers.Oncol Rep. 2013 Jan;29(1):141-8. doi: 10.3892/or.2012.2088. Epub 2012 Oct 17. Oncol Rep. 2013. PMID: 23076599 Clinical Trial.
-
CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production.Sci Rep. 2019 Oct 10;9(1):14611. doi: 10.1038/s41598-019-51149-1. Sci Rep. 2019. PMID: 31601953 Free PMC article.
-
Clinicopathologic and prognostic significance of tumor-associated macrophages in patients with hepatocellular carcinoma: A meta-analysis.PLoS One. 2019 Oct 16;14(10):e0223971. doi: 10.1371/journal.pone.0223971. eCollection 2019. PLoS One. 2019. Retraction in: PLoS One. 2023 Feb 17;18(2):e0282201. doi: 10.1371/journal.pone.0282201. PMID: 31618252 Free PMC article. Retracted.
-
Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis.Oral Oncol. 2019 Jun;93:66-75. doi: 10.1016/j.oraloncology.2019.04.019. Epub 2019 Apr 28. Oral Oncol. 2019. PMID: 31109698 Review.
-
Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer.Cancer Lett. 2013 May 10;332(1):3-10. doi: 10.1016/j.canlet.2013.01.024. Epub 2013 Jan 21. Cancer Lett. 2013. PMID: 23348699 Review.
Cited by
-
Spatial N-glycomics of the normal breast microenvironment reveals fucosylated and high-mannose N-glycan signatures related to BI-RADS density and ancestry.Glycobiology. 2024 Jun 22;34(8):cwae043. doi: 10.1093/glycob/cwae043. Glycobiology. 2024. PMID: 38869882
-
The Role of Chronic Inflammation in the Development of Breast Cancer.Cancers (Basel). 2021 Aug 3;13(15):3918. doi: 10.3390/cancers13153918. Cancers (Basel). 2021. PMID: 34359821 Free PMC article. Review.
-
Effects of Adiposity and Exercise on Breast Tissue and Systemic Metabo-Inflammatory Factors in Women at High Risk or Diagnosed with Breast Cancer.Cancer Prev Res (Phila). 2021 May;14(5):541-550. doi: 10.1158/1940-6207.CAPR-20-0507. Epub 2021 Mar 1. Cancer Prev Res (Phila). 2021. PMID: 33648942 Free PMC article.
-
The Role of Diet in Cancer Prevention and Chemotherapy Efficacy.Annu Rev Nutr. 2020 Sep 23;40:273-297. doi: 10.1146/annurev-nutr-013120-041149. Epub 2020 Jun 16. Annu Rev Nutr. 2020. PMID: 32543948 Free PMC article. Review.
-
Cancer-associated adipocytes: key players in breast cancer progression.J Hematol Oncol. 2019 Sep 10;12(1):95. doi: 10.1186/s13045-019-0778-6. J Hematol Oncol. 2019. PMID: 31500658 Free PMC article. Review.
References
-
- Cancer Facts and Figures 2014. American Cancer Society; Atlanta: 2014.
-
- Porter PL, Lund MJ, Lin MG, Yuan X, Liff JM, Flagg EW, Coates RJ, Eley JW. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100(12):2533–2542. doi:10.1002/cncr.20279. - PubMed
-
- Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer. 2004;4(7):519–527. doi:10.1038/nrc1389. - PubMed
-
- Amend K, Hicks D, Ambrosone CB. Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Res. 2006;66(17):8327–8330. doi:10.1158/0008-5472.CAN-06-1927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

