Targeted Delivery of Deoxycytidine Kinase to Her2-Positive Cells Enhances the Efficacy of the Nucleoside Analog Fludarabine
- PMID: 27280468
- PMCID: PMC4900609
- DOI: 10.1371/journal.pone.0157114
Targeted Delivery of Deoxycytidine Kinase to Her2-Positive Cells Enhances the Efficacy of the Nucleoside Analog Fludarabine
Abstract
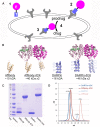
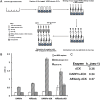
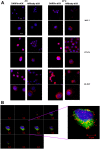
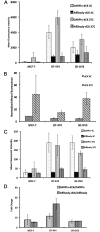
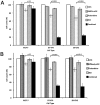
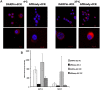
Cytotoxic drugs, such as nucleoside analogs and toxins, commonly suffer from off-target effects. One approach to mitigate this problem is to deliver the cytotoxic drug selectively to the intended site. While for toxins this can be achieved by conjugating the cell-killing moiety to a targeting moiety, it is not an option for nucleoside analogs, which rely on intracellular enzymes to convert them to their active triphosphorylated form. To overcome this limitation, and achieve site-targeted activation of nucleoside analogs, we fused the coding region of a prodrug-activating enzyme, deoxycytidine kinase (dCK), to affinity reagents that bind to the Her2 cell surface protein. We evaluated dCK fusions to an anti-Her2 affibody and Designed Ankyrin Repeat Protein (DARPin) for their ability to kill cancer cells by promoting the activation of the nucleoside analog fludarabine. Cell staining and flow cytometry experiments with three Her2 positive cancer cell lines (BT-474-JB, JIMT-1 and SK-OV-3) indicate dCK fusions binding and cellular internalization. In contrast, these reagents bind only weakly to the Her2 negative cell line, MCF-7. Cell proliferation assays indicate that SK-OV-3 and BT-474-JB cell lines exhibit significantly reduced proliferation rates when treated with targeting-module fused dCK and fludarabine, compared to fludarabine alone. These findings demonstrate that we have succeeded in delivering active dCK into the Her2-positive cells, thereby increasing the activation of fludarabine, which ultimately reduces the dose of nucleoside analog needed for cell killing. This strategy may help establish the therapeutic index required to differentiate between healthy tissues and cancer cells.
Conflict of interest statement
Figures
Similar articles
-
Retroviral transfer of deoxycytidine kinase into tumor cell lines enhances nucleoside toxicity.Cancer Res. 1996 May 15;56(10):2343-7. Cancer Res. 1996. PMID: 8625309
-
Phosphorylation of deoxycytidine kinase on Ser-74: impact on kinetic properties and nucleoside analog activation in cancer cells.Biochem Pharmacol. 2012 Jul 1;84(1):43-51. doi: 10.1016/j.bcp.2012.03.022. Epub 2012 Apr 2. Biochem Pharmacol. 2012. PMID: 22490700
-
Deoxycytidine kinase is overexpressed in poor outcome breast cancer and determines responsiveness to nucleoside analogs.Breast Cancer Res Treat. 2012 Feb;131(3):809-18. doi: 10.1007/s10549-011-1477-3. Epub 2011 Apr 5. Breast Cancer Res Treat. 2012. PMID: 21465168
-
Cellular and clinical pharmacology of fludarabine.Clin Pharmacokinet. 2002;41(2):93-103. doi: 10.2165/00003088-200241020-00002. Clin Pharmacokinet. 2002. PMID: 11888330 Review.
-
New insights into the synergism of nucleoside analogs with radiotherapy.Radiat Oncol. 2013 Sep 26;8:223. doi: 10.1186/1748-717X-8-223. Radiat Oncol. 2013. PMID: 24066967 Free PMC article. Review.
Cited by
-
A Conjugate Based on Anti-HER2 Diaffibody and Auristatin E Targets HER2-Positive Cancer Cells.Int J Mol Sci. 2017 Feb 14;18(2):401. doi: 10.3390/ijms18020401. Int J Mol Sci. 2017. PMID: 28216573 Free PMC article.
References
-
- Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002;3:415–24. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

