Magnetofection Enhances Adenoviral Vector-based Gene Delivery in Skeletal Muscle Cells
- PMID: 27274908
- PMCID: PMC4888903
- DOI: 10.4172/2157-7439.1000364
Magnetofection Enhances Adenoviral Vector-based Gene Delivery in Skeletal Muscle Cells
Abstract
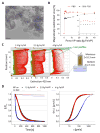
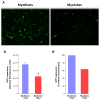
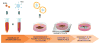
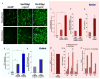
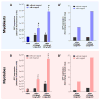
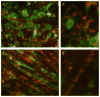
The goal of magnetic field-assisted gene transfer is to enhance internalization of exogenous nucleic acids by association with magnetic nanoparticles (MNPs). This technique named magnetofection is particularly useful in difficult-to-transfect cells. It is well known that human, mouse, and rat skeletal muscle cells suffer a maturation-dependent loss of susceptibility to Recombinant Adenoviral vector (RAd) uptake. In postnatal, fully differentiated myofibers, the expression of the primary Coxsackie and Adenoviral membrane receptor (CAR) is severely downregulated representing a main hurdle for the use of these vectors in gene transfer/therapy. Here we demonstrate that assembling of Recombinant Adenoviral vectors with suitable iron oxide MNPs into magneto-adenovectors (RAd-MNP) and further exposure to a gradient magnetic field enables to efficiently overcome transduction resistance in skeletal muscle cells. Expression of Green Fluorescent Protein and Insulin-like Growth Factor 1 was significantly enhanced after magnetofection with RAd-MNPs complexes in C2C12 myotubes in vitro and mouse skeletal muscle in vivo when compared to transduction with naked virus. These results provide evidence that magnetofection, mainly due to its membrane-receptor independent mechanism, constitutes a simple and effective alternative to current methods for gene transfer into traditionally hard-to-transfect biological models.
Keywords: Adenoviral vectors; Gene delivery; Magnetic nanoparticles; Magneto-adenovectors; Magnetofection; Skeletal muscle.
Figures


Similar articles
-
Magnetically responsive biodegradable nanoparticles enhance adenoviral gene transfer in cultured smooth muscle and endothelial cells.Mol Pharm. 2009 Sep-Oct;6(5):1380-7. doi: 10.1021/mp900017m. Mol Pharm. 2009. PMID: 19496618 Free PMC article.
-
Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells.Hum Gene Ther. 1999 Apr 10;10(6):1009-19. doi: 10.1089/10430349950018409. Hum Gene Ther. 1999. PMID: 10223734
-
Adenovirus vectors based on human adenovirus type 19a have high potential for human muscle-directed gene therapy.Hum Gene Ther. 2006 Feb;17(2):193-205. doi: 10.1089/hum.2006.17.193. Hum Gene Ther. 2006. PMID: 16454653
-
Magnetic field-assisted gene delivery: achievements and therapeutic potential.Curr Gene Ther. 2012 Apr 1;12(2):116-26. doi: 10.2174/156652312800099616. Curr Gene Ther. 2012. PMID: 22348552 Review.
-
Magnetically-assisted viral transduction (magnetofection) medical applications: An update.Biomater Adv. 2023 Nov;154:213657. doi: 10.1016/j.bioadv.2023.213657. Epub 2023 Oct 14. Biomater Adv. 2023. PMID: 37844415 Review.
Cited by
-
Advanced physical techniques for gene delivery based on membrane perforation.Drug Deliv. 2018 Nov;25(1):1516-1525. doi: 10.1080/10717544.2018.1480674. Drug Deliv. 2018. PMID: 29968512 Free PMC article. Review.
-
Superparamagnetic Iron Oxide Nanoparticles in Musculoskeletal Biology.Tissue Eng Part B Rev. 2017 Aug;23(4):373-385. doi: 10.1089/ten.TEB.2016.0437. Epub 2017 Jan 11. Tissue Eng Part B Rev. 2017. PMID: 27998240 Free PMC article.
-
Biopolymers augment viral vectors based gene delivery.J Biosci. 2019 Sep;44(4):84. J Biosci. 2019. PMID: 31502562 Review.
References
-
- Widder KJ, Senyel AE, Scarpelli GD. Magnetic microspheres: a model system of site specific drug delivery in vivo. Proc Soc Exp Biol Med. 1978;158:141–146. - PubMed
-
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. - PubMed
-
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. - PubMed
-
- Tresilwised N, Pithayanukul P, Holm PS, Schillinger U, Plank C, et al. Effects of nanoparticle coatings on the activity of oncolytic adenovirus-magnetic nanoparticle complexes. Biomaterials. 2012;33:256–269. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
