GAP junctional communication in brain secondary organizers
- PMID: 27273333
- PMCID: PMC11520981
- DOI: 10.1111/dgd.12297
GAP junctional communication in brain secondary organizers
Abstract
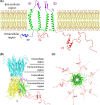
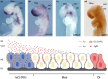
Gap junctions (GJs) are integral membrane proteins that enable the direct cytoplasmic exchange of ions and low molecular weight metabolites between adjacent cells. They are formed by the apposition of two connexons belonging to adjacent cells. Each connexon is formed by six proteins, named connexins (Cxs). Current evidence suggests that gap junctions play an important part in ensuring normal embryo development. Mutations in connexin genes have been linked to a variety of human diseases, although the precise role and the cell biological mechanisms of their action remain almost unknown. Among the big family of Cxs, several are expressed in nervous tissue but just a few are expressed in the anterior neural tube of vertebrates. Many efforts have been made to elucidate the molecular bases of Cxs cell biology and how they influence the morphogenetic signal activity produced by brain signaling centers. These centers, orchestrated by transcription factors and morphogenes determine the axial patterning of the mammalian brain during its specification and regionalization. The present review revisits the findings of GJ composed by Cx43 and Cx36 in neural tube patterning and discuss Cx43 putative enrollment in the control of Fgf8 signal activity coming from the well known secondary organizer, the isthmic organizer.
Keywords: Cx36; Cx43; Fgf8; gap junction; isthmic organizer; mammalian neural tube patterning; morphogenesis.
© 2016 The Authors. Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Figures
Similar articles
-
Functional analysis of hemichannels and gap-junctional channels formed by connexins 43 and 46.Mol Vis. 2010 Jul 15;16:1343-52. Mol Vis. 2010. PMID: 20664797 Free PMC article.
-
Gap junctional communication promotes apoptosis in a connexin-type-dependent manner.Cell Death Dis. 2013 Apr 11;4(4):e584. doi: 10.1038/cddis.2013.105. Cell Death Dis. 2013. PMID: 23579271 Free PMC article.
-
Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1.Mol Biol Cell. 2011 May;22(9):1516-28. doi: 10.1091/mbc.E10-06-0548. Epub 2011 Mar 16. Mol Biol Cell. 2011. PMID: 21411628 Free PMC article.
-
[Correlation of connexin 43 with testicular tumors].Zhonghua Nan Ke Xue. 2017 Mar;23(3):267-270. Zhonghua Nan Ke Xue. 2017. PMID: 29706050 Review. Chinese.
-
Expression pattern of different gap junction connexins is related to embryo implantation.Int J Dev Biol. 1996 Feb;40(1):361-7. Int J Dev Biol. 1996. PMID: 8735949 Review.
Cited by
-
Connexin Channels at the Glio-Vascular Interface: Gatekeepers of the Brain.Neurochem Res. 2017 Sep;42(9):2519-2536. doi: 10.1007/s11064-017-2313-x. Epub 2017 Jun 20. Neurochem Res. 2017. PMID: 28634726 Review.
-
Lactate as a Metabolite and a Regulator in the Central Nervous System.Int J Mol Sci. 2016 Sep 1;17(9):1450. doi: 10.3390/ijms17091450. Int J Mol Sci. 2016. PMID: 27598136 Free PMC article. Review.
-
Cell-Cell Connection Enhances Proliferation and Neuronal Differentiation of Rat Embryonic Neural Stem/Progenitor Cells.Front Cell Neurosci. 2017 Jul 21;11:200. doi: 10.3389/fncel.2017.00200. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28785204 Free PMC article.
-
The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D.Cell Metab. 2017 Nov 7;26(5):719-737.e6. doi: 10.1016/j.cmet.2017.08.024. Epub 2017 Sep 28. Cell Metab. 2017. PMID: 28965825 Free PMC article.
-
An Update on the Molecular Mechanism of the Vertebrate Isthmic Organizer Development in the Context of the Neuromeric Model.Front Neuroanat. 2022 Mar 24;16:826976. doi: 10.3389/fnana.2022.826976. eCollection 2022. Front Neuroanat. 2022. PMID: 35401126 Free PMC article. Review.
References
-
- Allison, D. W. , Ohran, A. J. , Stobbs, S. H. , Mameli, M. , Valenzuela, C. F. , Sudweeks, S. N. , Ray, A. P. , Henriksen, S. J. & Steffensen, S. C. 2006. Connexin‐36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse. 60, 20–31. - PubMed
-
- Bai, D. 2016. Structural analysis of key gap junction domains‐Lessons from genome data and disease‐linked mutants. Semin. Cell Dev. Biol. 50, 74–82. - PubMed
-
- Bergoffen, J. , Scherer, S. S. , Wang, S. , Scott, M. O. , Bone, L. J. , Paul, D. L. , Chen, K. , Lensch, M. W. , Chance, P. F. & Fischbeck, K. H. 1993. Connexin mutations in X‐linked Charcot‐Marie‐Tooth disease. Science 262, 2039–2042. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

