Expression of IL-1β in rhesus EAE and MS lesions is mainly induced in the CNS itself
- PMID: 27266875
- PMCID: PMC4895983
- DOI: 10.1186/s12974-016-0605-8
Expression of IL-1β in rhesus EAE and MS lesions is mainly induced in the CNS itself
Abstract
Background: Interleukin (IL)-1β is a pro-inflammatory cytokine that plays a role in the pathogenesis of multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE), the animal model for MS. Yet, detailed studies on IL-1β expression in different stages of MS lesion development and a comparison of IL-1β expression in MS and EAE are lacking.
Methods: Here, we performed an extensive characterization of IL-1β expression in brain tissue of MS patients, which included different MS lesion types, and in brain tissue of rhesus macaques with EAE.
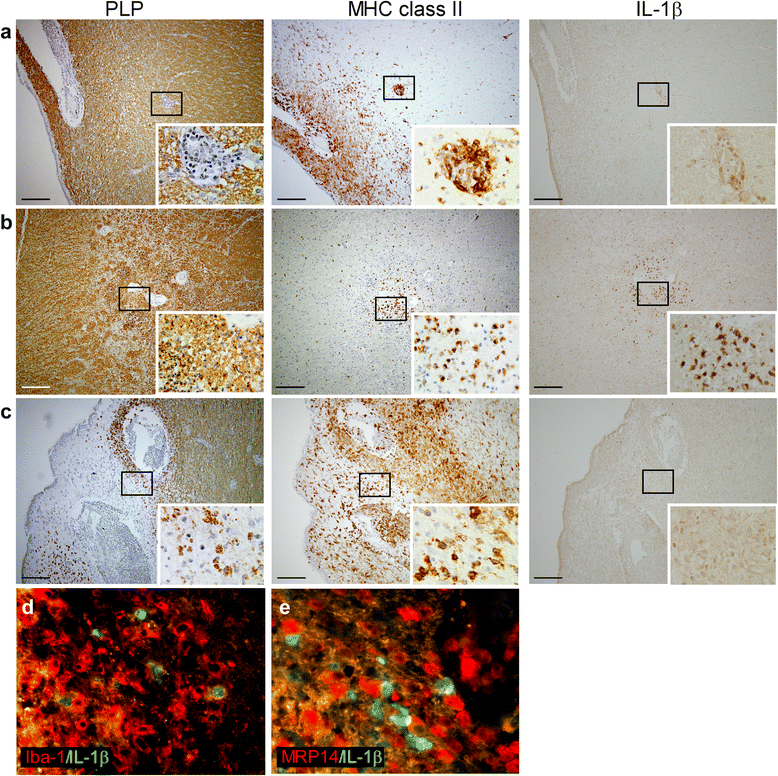
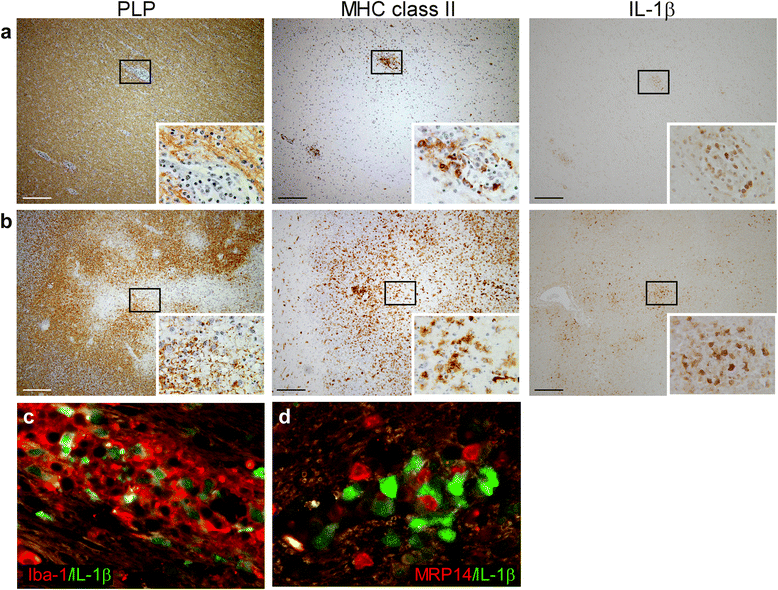
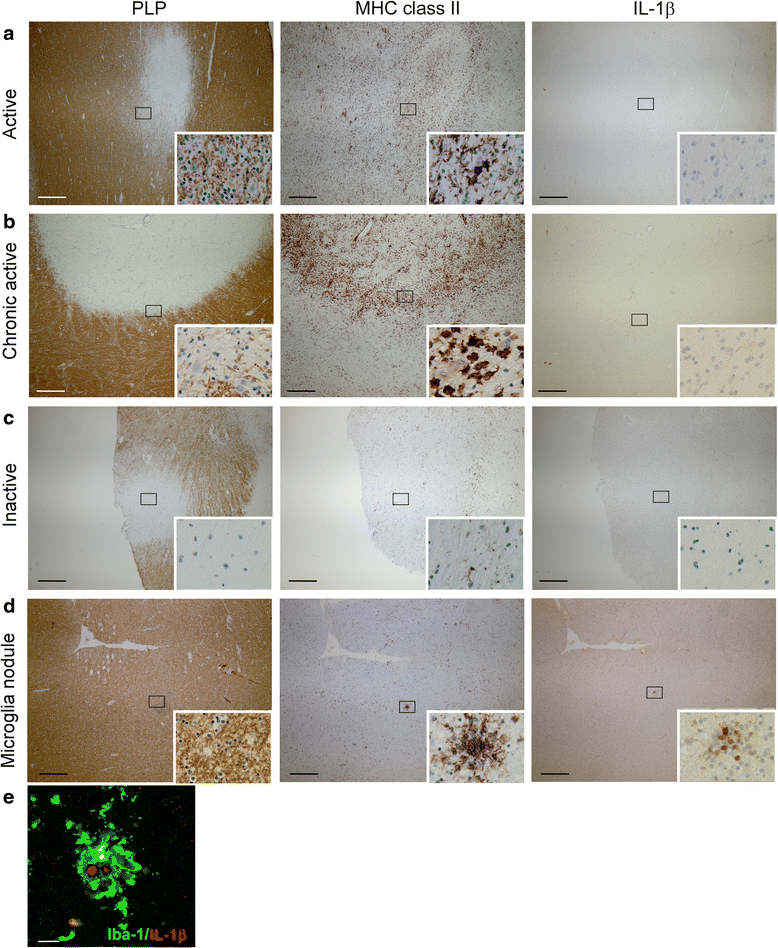
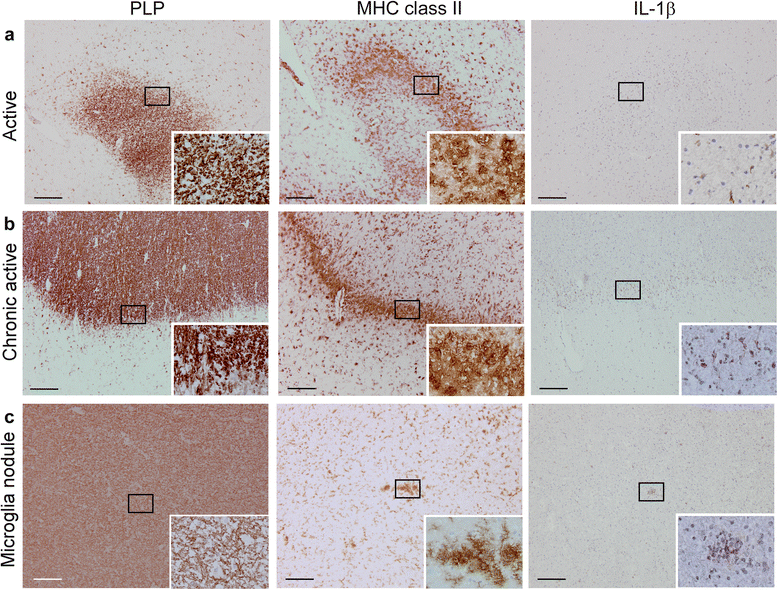
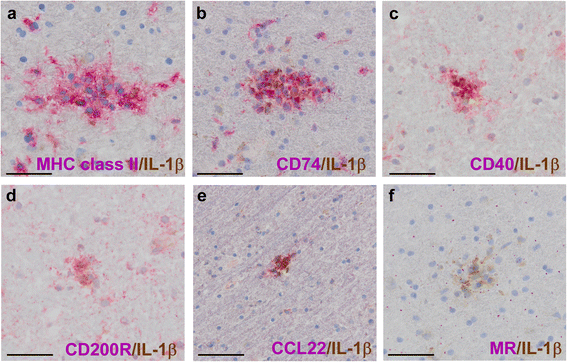
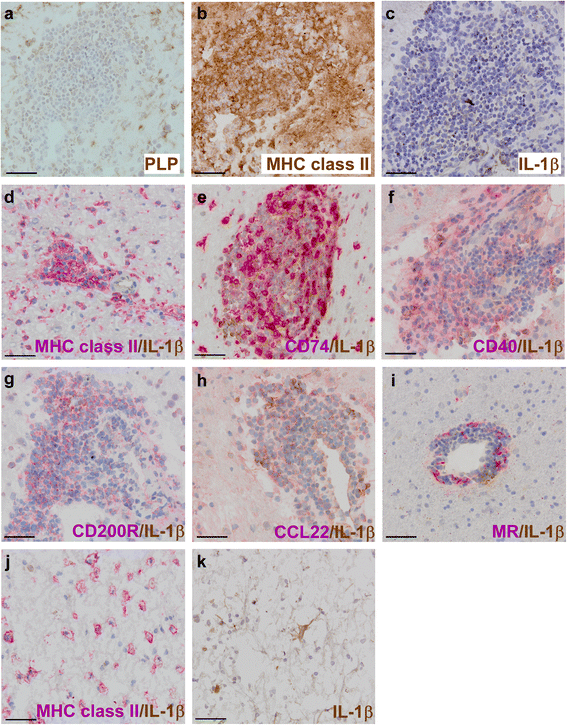
Results: In rhesus EAE brain tissue, we observed prominent IL-1β staining in MHC class II(+) cells within perivascular infiltrates and at the edges of large demyelinating lesions. Surprisingly, staining was localized to resident microglia or differentiated macrophages rather than to infiltrating monocytes, suggesting that IL-1β expression is induced within the central nervous system (CNS). By contrast, IL-1β staining in MS brain tissue was much less pronounced. Staining was found in the parenchyma of active and chronic active MS lesions and in nodules of MHC class II(+) microglia in otherwise normal appearing white matter. IL-1β expression was detected in a minority of the nodules only, which could not be distinguished by the expression of pro- and anti-inflammatory markers. These nodules were exclusively found in MS, and it remains to be determined whether IL-1β(+) nodules are destined to progress into active lesions or whether they merely reflect a transient response to cellular stress.
Conclusions: Although the exact localization and relative intensity of IL-1β expression in EAE and MS is different, the staining pattern in both neuroinflammatory disorders is most consistent with the idea that the expression of IL-1β during lesion development is induced in the tissue rather than in the periphery.
Keywords: Experimental autoimmune encephalomyelitis; IL-1β; Inflammasome; Microglia; Multiple sclerosis; Preactive lesion.
Figures
Similar articles
-
Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis.Brain Behav Immun. 2010 May;24(4):641-51. doi: 10.1016/j.bbi.2010.01.014. Epub 2010 Feb 6. Brain Behav Immun. 2010. PMID: 20138983
-
Temporal expression and cellular origin of CC chemokine receptors CCR1, CCR2 and CCR5 in the central nervous system: insight into mechanisms of MOG-induced EAE.J Neuroinflammation. 2007 May 7;4:14. doi: 10.1186/1742-2094-4-14. J Neuroinflammation. 2007. PMID: 17484785 Free PMC article.
-
In acute experimental autoimmune encephalomyelitis, infiltrating macrophages are immune activated, whereas microglia remain immune suppressed.Glia. 2014 Oct;62(10):1724-35. doi: 10.1002/glia.22711. Epub 2014 Jun 23. Glia. 2014. PMID: 24953459
-
New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis.J Immunol. 2017 Jun 15;198(12):4553-4560. doi: 10.4049/jimmunol.1700263. J Immunol. 2017. PMID: 28583987 Free PMC article. Review.
-
IL-1β dependent cerebellar synaptopathy in a mouse mode of multiple sclerosis.Cerebellum. 2015 Feb;14(1):19-22. doi: 10.1007/s12311-014-0613-0. Cerebellum. 2015. PMID: 25326653 Review.
Cited by
-
Role of Inflammasomes in Neuroimmune and Neurodegenerative Diseases: A Systematic Review.Mediators Inflamm. 2018 Apr 17;2018:1549549. doi: 10.1155/2018/1549549. eCollection 2018. Mediators Inflamm. 2018. PMID: 29849483 Free PMC article. Review.
-
DNA methylation changes in glial cells of the normal-appearing white matter in Multiple Sclerosis patients.Epigenetics. 2022 Nov;17(11):1311-1330. doi: 10.1080/15592294.2021.2020436. Epub 2022 Jan 30. Epigenetics. 2022. PMID: 35094644 Free PMC article.
-
Dopamine activates NF-κB and primes the NLRP3 inflammasome in primary human macrophages.Brain Behav Immun Health. 2020 Feb;2:100030. doi: 10.1016/j.bbih.2019.100030. Epub 2019 Dec 31. Brain Behav Immun Health. 2020. PMID: 33665636 Free PMC article.
-
Profiling of microglia nodules in multiple sclerosis reveals propensity for lesion formation.Nat Commun. 2024 Feb 23;15(1):1667. doi: 10.1038/s41467-024-46068-3. Nat Commun. 2024. PMID: 38396116 Free PMC article.
-
Mechanistic underpinning of an inside-out concept for autoimmunity in multiple sclerosis.Ann Clin Transl Neurol. 2021 Aug;8(8):1709-1719. doi: 10.1002/acn3.51401. Epub 2021 Jun 22. Ann Clin Transl Neurol. 2021. PMID: 34156169 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

