Molecular Pathways: Endothelial Cell FAK-A Target for Cancer Treatment
- PMID: 27262114
- PMCID: PMC5386133
- DOI: 10.1158/1078-0432.CCR-14-2021
Molecular Pathways: Endothelial Cell FAK-A Target for Cancer Treatment
Abstract
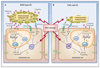
The nonreceptor protein tyrosine kinase, focal adhesion kinase (FAK, also known as PTK2), is a key mediator of signal transduction downstream of integrins and growth factor receptors in a variety of cells, including endothelial cells. FAK is upregulated in several advanced-stage solid tumors and has been described to promote tumor progression and metastasis through effects on both tumor cells and stromal cells. This observation has led to the development of several FAK inhibitors, some of which have entered clinical trials (GSK2256098, VS-4718, VS-6062, VS-6063, and BI853520). Resistance to chemotherapy is a serious limitation of cancer treatment and, until recently, most studies were restricted to tumor cells, excluding the possible roles performed by the tumor microenvironment. A recent report identified endothelial cell FAK (EC-FAK) as a major regulator of chemosensitivity. By dysregulating endothelial cell-derived paracrine (also known as angiocrine) signals, loss of FAK solely in the endothelial cell compartment is able to induce chemosensitization to DNA-damaging therapies in the malignant cell compartment and thereby reduce tumor growth. Herein, we summarize the roles of EC-FAK in cancer and development and review the status of FAK-targeting anticancer strategies. Clin Cancer Res; 22(15); 3718-24. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy.Nature. 2014 Oct 2;514(7520):112-6. doi: 10.1038/nature13541. Epub 2014 Jul 27. Nature. 2014. PMID: 25079333 Free PMC article.
-
Elucidating the role of the kinase activity of endothelial cell focal adhesion kinase in angiocrine signalling and tumour growth.J Pathol. 2022 Feb;256(2):235-247. doi: 10.1002/path.5833. Epub 2021 Dec 20. J Pathol. 2022. PMID: 34743335
-
Focal adhesion kinase signaling - tumor vulnerabilities and clinical opportunities.J Cell Sci. 2024 Jul 15;137(14):jcs261723. doi: 10.1242/jcs.261723. Epub 2024 Jul 22. J Cell Sci. 2024. PMID: 39034922 Free PMC article. Review.
-
Oncogenic Receptor Tyrosine Kinases Directly Phosphorylate Focal Adhesion Kinase (FAK) as a Resistance Mechanism to FAK-Kinase Inhibitors.Mol Cancer Ther. 2016 Dec;15(12):3028-3039. doi: 10.1158/1535-7163.MCT-16-0366. Epub 2016 Sep 16. Mol Cancer Ther. 2016. PMID: 27638858 Free PMC article.
-
The roles of nuclear focal adhesion kinase (FAK) on Cancer: a focused review.J Exp Clin Cancer Res. 2019 Jun 11;38(1):250. doi: 10.1186/s13046-019-1265-1. J Exp Clin Cancer Res. 2019. PMID: 31186061 Free PMC article. Review.
Cited by
-
Epithelial-mesenchymal transition and metastasis of colon cancer cells induced by the FAK pathway in cancer-associated fibroblasts.J Int Med Res. 2020 Jun;48(6):300060520931242. doi: 10.1177/0300060520931242. J Int Med Res. 2020. PMID: 32588696 Free PMC article.
-
Genistein Inhibits Proliferation and Metastasis in Human Cervical Cancer Cells through the Focal Adhesion Kinase Signaling Pathway: A Network Pharmacology-Based In Vitro Study in HeLa Cells.Molecules. 2023 Feb 17;28(4):1919. doi: 10.3390/molecules28041919. Molecules. 2023. PMID: 36838908 Free PMC article.
-
TPMT mRNA Expression: A Novel Prognostic Biomarker for Patients with Colon Cancer by Bioinformatics Analysis.Int J Gen Med. 2022 Jan 6;15:151-160. doi: 10.2147/IJGM.S338575. eCollection 2022. Int J Gen Med. 2022. PMID: 35023953 Free PMC article.
-
Small Ones to Fight a Big Problem-Intervention of Cancer Metastasis by Small Molecules.Cancers (Basel). 2020 Jun 3;12(6):1454. doi: 10.3390/cancers12061454. Cancers (Basel). 2020. PMID: 32503267 Free PMC article. Review.
-
Functional and clinical characteristics of focal adhesion kinases in cancer progression.Front Cell Dev Biol. 2022 Nov 2;10:1040311. doi: 10.3389/fcell.2022.1040311. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36407100 Free PMC article. Review.
References
-
- Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–74. - PubMed
-
- McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–15. - PubMed
-
- van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. - PubMed
-
- Lechertier T, Hodivala-Dilke K. Focal adhesion kinase and tumour angiogenesis. J Pathol. 2012;226:404–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

