Meckel's and condylar cartilages anomalies in achondroplasia result in defective development and growth of the mandible
- PMID: 27260401
- PMCID: PMC5181594
- DOI: 10.1093/hmg/ddw153
Meckel's and condylar cartilages anomalies in achondroplasia result in defective development and growth of the mandible
Abstract
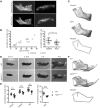
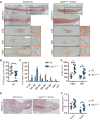
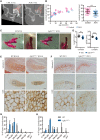
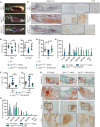
Activating FGFR3 mutations in human result in achondroplasia (ACH), the most frequent form of dwarfism, where cartilages are severely disturbed causing long bones, cranial base and vertebrae defects. Because mandibular development and growth rely on cartilages that guide or directly participate to the ossification process, we investigated the impact of FGFR3 mutations on mandibular shape, size and position. By using CT scan imaging of ACH children and by analyzing Fgfr3Y367C/+ mice, a model of ACH, we show that FGFR3 gain-of-function mutations lead to structural anomalies of primary (Meckel's) and secondary (condylar) cartilages of the mandible, resulting in mandibular hypoplasia and dysmorphogenesis. These defects are likely related to a defective chondrocyte proliferation and differentiation and pan-FGFR tyrosine kinase inhibitor NVP-BGJ398 corrects Meckel's and condylar cartilages defects ex vivo. Moreover, we show that low dose of NVP-BGJ398 improves in vivo condyle growth and corrects dysmorphologies in Fgfr3Y367C/+ mice, suggesting that postnatal treatment with NVP-BGJ398 mice might offer a new therapeutic strategy to improve mandible anomalies in ACH and others FGFR3-related disorders.
© The Author 2016. Published by Oxford University Press.
Figures


Similar articles
-
Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model.J Clin Invest. 2016 May 2;126(5):1871-84. doi: 10.1172/JCI83926. Epub 2016 Apr 11. J Clin Invest. 2016. PMID: 27064282 Free PMC article.
-
A novel tyrosine kinase inhibitor restores chondrocyte differentiation and promotes bone growth in a gain-of-function Fgfr3 mouse model.Hum Mol Genet. 2012 Feb 15;21(4):841-51. doi: 10.1093/hmg/ddr514. Epub 2011 Nov 9. Hum Mol Genet. 2012. PMID: 22072392
-
FGFR3 mutation causes abnormal membranous ossification in achondroplasia.Hum Mol Genet. 2014 Jun 1;23(11):2914-25. doi: 10.1093/hmg/ddu004. Epub 2014 Jan 12. Hum Mol Genet. 2014. PMID: 24419316
-
C-Type Natriuretic Peptide Analog as Therapy for Achondroplasia.Endocr Dev. 2016;30:98-105. doi: 10.1159/000439334. Epub 2015 Dec 10. Endocr Dev. 2016. PMID: 26684019 Review.
-
Achondroplasia: from genotype to phenotype.Joint Bone Spine. 2008 Mar;75(2):125-30. doi: 10.1016/j.jbspin.2007.06.007. Epub 2007 Sep 25. Joint Bone Spine. 2008. PMID: 17950653 Review.
Cited by
-
Mandibular dysmorphology due to abnormal embryonic osteogenesis in FGFR2-related craniosynostosis mice.Dis Model Mech. 2019 May 30;12(5):dmm038513. doi: 10.1242/dmm.038513. Dis Model Mech. 2019. PMID: 31064775 Free PMC article.
-
Overexpression of Fgf18 in cranial neural crest cells recapitulates Pierre Robin sequence in mice.Front Cell Dev Biol. 2024 Apr 17;12:1376814. doi: 10.3389/fcell.2024.1376814. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38694818 Free PMC article.
-
Advantages and Disadvantages of Different Treatment Methods in Achondroplasia: A Review.Int J Mol Sci. 2021 May 25;22(11):5573. doi: 10.3390/ijms22115573. Int J Mol Sci. 2021. PMID: 34070375 Free PMC article. Review.
-
Axolotl mandible regeneration occurs through mechanical gap closure and a shared regenerative program with the limb.Dis Model Mech. 2024 Sep 1;17(9):dmm050743. doi: 10.1242/dmm.050743. Epub 2024 Sep 27. Dis Model Mech. 2024. PMID: 39206627 Free PMC article.
-
Reconstruction of the temporomandibular joint using a vascularized medial femoral condyle osteocartilaginous flap: an experimental investigation in miniature pigs.BMC Oral Health. 2023 Sep 1;23(1):621. doi: 10.1186/s12903-023-03341-z. BMC Oral Health. 2023. PMID: 37658390 Free PMC article.
References
-
- Baujat G., Legeai-Mallet L., Finidori G., Cormier-Daire V., Le Merrer M. (2008) Achondroplasia. Best Pract. Res. Clin. Rheumatol, 22, 3–18. - PubMed
-
- Horton W.A., Hall J.G., Hecht J.T. (2007) Achondroplasia. Lancet, 370, 162–172. - PubMed
-
- Di Rocco F., Biosse Duplan M., Heuzé Y., Kaci N., Komla-Ebri D., Munnich A., Mugniery E., Benoist-Lasselin C., Legeai-Mallet L. (2014) FGFR3 mutation causes abnormal membranous ossification in achondroplasia. Hum. Mol. Genet, 23, 2914–2925. - PubMed
-
- Rousseau F., Bonaventure J., Legeai-Mallet L., Pelet A., Rozet J.M., Maroteaux P., Le Merrer M., Munnich A. (1994) Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature, 371, 252–254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

