Endogenous Mouse Dicer Is an Exclusively Cytoplasmic Protein
- PMID: 27254021
- PMCID: PMC4890738
- DOI: 10.1371/journal.pgen.1006095
Endogenous Mouse Dicer Is an Exclusively Cytoplasmic Protein
Abstract
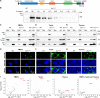
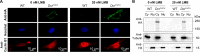
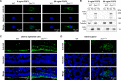
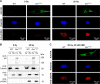
Dicer is a large multi-domain protein responsible for the ultimate step of microRNA and short-interfering RNA biogenesis. In human and mouse cell lines, Dicer has been shown to be important in the nuclear clearance of dsRNA as well as the establishment of chromatin modifications. Here we set out to unambiguously define the cellular localization of Dicer in mice to understand if this is a conserved feature of mammalian Dicer in vivo. To this end, we utilized an endogenously epitope tagged Dicer knock-in mouse allele. From primary mouse cell lines and adult tissues, we determined with certainty by biochemical fractionation and confocal immunofluorescence microscopy that endogenous Dicer is exclusively cytoplasmic. We ruled out the possibility that a fraction of Dicer shuttles to and from the nucleus as well as that FGF or DNA damage signaling induce Dicer nuclear translocation. We also explored Dicer localization during the dynamic and developmental context of embryogenesis, where Dicer is ubiquitously expressed and strictly cytoplasmic in all three germ layers as well as extraembryonic tissues. Our data exclude a direct role for Dicer in the nuclear RNA processing in the mouse.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Nuclear re-localization of Dicer in primary mouse embryonic fibroblast nuclei following DNA damage.PLoS Genet. 2018 Feb 2;14(2):e1007151. doi: 10.1371/journal.pgen.1007151. eCollection 2018 Feb. PLoS Genet. 2018. PMID: 29394246 Free PMC article.
-
Efficient Dicer processing of virus-derived double-stranded RNAs and its modulation by RIG-I-like receptor LGP2.PLoS Pathog. 2021 Aug 3;17(8):e1009790. doi: 10.1371/journal.ppat.1009790. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34343211 Free PMC article.
-
Nuclear phosphorylated Dicer processes double-stranded RNA in response to DNA damage.J Cell Biol. 2017 Aug 7;216(8):2373-2389. doi: 10.1083/jcb.201612131. Epub 2017 Jun 22. J Cell Biol. 2017. PMID: 28642363 Free PMC article.
-
Plant dicer-like proteins: double-stranded RNA-cleaving enzymes for small RNA biogenesis.J Plant Res. 2017 Jan;130(1):33-44. doi: 10.1007/s10265-016-0877-1. Epub 2016 Nov 24. J Plant Res. 2017. PMID: 27885504 Review.
-
Molecular mechanisms of Dicer: endonuclease and enzymatic activity.Biochem J. 2017 May 4;474(10):1603-1618. doi: 10.1042/BCJ20160759. Biochem J. 2017. PMID: 28473628 Free PMC article. Review.
Cited by
-
Restricted and non-essential redundancy of RNAi and piRNA pathways in mouse oocytes.PLoS Genet. 2019 Dec 20;15(12):e1008261. doi: 10.1371/journal.pgen.1008261. eCollection 2019 Dec. PLoS Genet. 2019. PMID: 31860668 Free PMC article.
-
NANOS2 is a sequence-specific mRNA-binding protein that promotes transcript degradation in spermatogonial stem cells.iScience. 2021 Jun 24;24(7):102762. doi: 10.1016/j.isci.2021.102762. eCollection 2021 Jul 23. iScience. 2021. PMID: 34278268 Free PMC article.
-
From "Cellular" RNA to "Smart" RNA: Multiple Roles of RNA in Genome Stability and Beyond.Chem Rev. 2018 Apr 25;118(8):4365-4403. doi: 10.1021/acs.chemrev.7b00487. Epub 2018 Mar 30. Chem Rev. 2018. PMID: 29600857 Free PMC article. Review.
-
DROSHA is recruited to DNA damage sites by the MRN complex to promote non-homologous end joining.J Cell Sci. 2021 Mar 22;134(6):jcs249706. doi: 10.1242/jcs.249706. J Cell Sci. 2021. PMID: 33558311 Free PMC article.
-
DICER regulates the expression of major satellite repeat transcripts and meiotic chromosome segregation during spermatogenesis.Nucleic Acids Res. 2020 Jul 27;48(13):7135-7153. doi: 10.1093/nar/gkaa460. Nucleic Acids Res. 2020. PMID: 32484548 Free PMC article.
References
-
- Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, et al. (2002) Prediction of plant microRNA targets. Cell 110: 513–520. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

