Vaccine-Elicited Mucosal and Systemic Antibody Responses Are Associated with Reduced Simian Immunodeficiency Viremia in Infant Rhesus Macaques
- PMID: 27252535
- PMCID: PMC4984660
- DOI: 10.1128/JVI.00481-16
Vaccine-Elicited Mucosal and Systemic Antibody Responses Are Associated with Reduced Simian Immunodeficiency Viremia in Infant Rhesus Macaques
Abstract
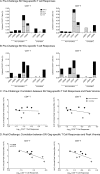
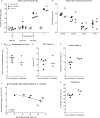
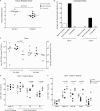
Despite significant progress in reducing peripartum mother-to-child transmission (MTCT) of human immunodeficiency virus (HIV) with antiretroviral therapy (ART), continued access to ART throughout the breastfeeding period is still a limiting factor, and breast milk exposure to HIV accounts for up to 44% of MTCT. As abstinence from breastfeeding is not recommended, alternative means are needed to prevent MTCT of HIV. We have previously shown that oral vaccination at birth with live attenuated Mycobacterium tuberculosis strains expressing simian immunodeficiency virus (SIV) genes safely induces persistent SIV-specific cellular and humoral immune responses both systemically and at the oral and intestinal mucosa. Here, we tested the ability of oral M. tuberculosis vaccine strains expressing SIV Env and Gag proteins, followed by systemic heterologous (MVA-SIV Env/Gag/Pol) boosting, to protect neonatal macaques against oral SIV challenge. While vaccination did not protect infant macaques against oral SIV acquisition, a subset of immunized animals had significantly lower peak viremia which inversely correlated with prechallenge SIV Env-specific salivary and intestinal IgA responses and higher-avidity SIV Env-specific IgG in plasma. These controller animals also maintained CD4(+) T cell populations better and showed reduced tissue pathology compared to noncontroller animals. We show that infants vaccinated at birth can develop vaccine-induced SIV-specific IgA and IgG antibodies and cellular immune responses within weeks of life. Our data further suggest that affinity maturation of vaccine-induced plasma antibodies and induction of mucosal IgA responses at potential SIV entry sites are associated with better control of viral replication, thereby likely reducing SIV morbidity.
Importance: Despite significant progress in reducing peripartum MTCT of HIV with ART, continued access to ART throughout the breastfeeding period is still a limiting factor. Breast milk exposure to HIV accounts for up to 44% of MTCT. Alternative measures, in addition to ART, are needed to achieve the goal of an AIDS-free generation. Pediatric HIV vaccines constitute a core component of such efforts. The results of our pediatric vaccine study highlight the potential importance of vaccine-elicited mucosal Env-specific IgA responses in combination with high-avidity systemic Env-specific IgG in protection against oral SIV transmission and control of viral replication in infant macaques. The induction of potent mucosal IgA antibodies by our vaccine is remarkable considering the age-dependent development of mucosal IgA responses postbirth. A deeper understanding of postnatal immune development may inform the design of improved vaccine strategies to enhance systemic and mucosal SIV/HIV antibody responses.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Virus-Like Particles Displaying Trimeric Simian Immunodeficiency Virus (SIV) Envelope gp160 Enhance the Breadth of DNA/Modified Vaccinia Virus Ankara SIV Vaccine-Induced Antibody Responses in Rhesus Macaques.J Virol. 2016 Sep 12;90(19):8842-54. doi: 10.1128/JVI.01163-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466414 Free PMC article.
-
CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge.J Virol. 2014 Sep 1;88(17):9579-89. doi: 10.1128/JVI.00975-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920805 Free PMC article.
-
Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques.Vaccine. 2011 Apr 12;29(17):3124-37. doi: 10.1016/j.vaccine.2011.02.051. Epub 2011 Mar 4. Vaccine. 2011. PMID: 21377510 Free PMC article.
-
Models of Protective Immunity against Schistosomes: Implications for Vaccine Development.Pathogens. 2023 Oct 3;12(10):1215. doi: 10.3390/pathogens12101215. Pathogens. 2023. PMID: 37887731 Free PMC article. Review.
-
A new hope? Possibilities of therapeutic IgA antibodies in the treatment of inflammatory lung diseases.Front Immunol. 2023 Mar 27;14:1127339. doi: 10.3389/fimmu.2023.1127339. eCollection 2023. Front Immunol. 2023. PMID: 37051237 Free PMC article. Review.
Cited by
-
Maternal HIV-1 Env Vaccination for Systemic and Breast Milk Immunity To Prevent Oral SHIV Acquisition in Infant Macaques.mSphere. 2018 Jan 10;3(1):e00505-17. doi: 10.1128/mSphere.00505-17. eCollection 2018 Jan-Feb. mSphere. 2018. PMID: 29359183 Free PMC article.
-
Antibody-mediated immune exclusion of HIV.Curr Opin HIV AIDS. 2017 May;12(3):222-228. doi: 10.1097/COH.0000000000000369. Curr Opin HIV AIDS. 2017. PMID: 28422786 Free PMC article. Review.
-
Dam-Infant Rhesus Macaque Pairs to Dissect Age-Dependent Responses to SARS-CoV-2 Infection.Immunohorizons. 2022 Dec 1;6(12):851-863. doi: 10.4049/immunohorizons.2200075. Immunohorizons. 2022. PMID: 36547390 Free PMC article.
-
Trained Immunity and Susceptibility to HIV.Clin Vaccine Immunol. 2017 Jan 5;24(1):e00509-16. doi: 10.1128/CVI.00509-16. Print 2017 Jan. Clin Vaccine Immunol. 2017. PMID: 27847369 Free PMC article.
-
Early Sites of Virus Replication After Oral SIVmac251 Infection of Infant Macaques: Implications for Pathogenesis.AIDS Res Hum Retroviruses. 2018 Mar;34(3):286-299. doi: 10.1089/AID.2017.0169. Epub 2018 Jan 17. AIDS Res Hum Retroviruses. 2018. PMID: 29237287 Free PMC article.
References
-
- WHO. 2014. Global update on the health sector response to HIV, 2014. World Health Organization, Geneva, Switzerland.
-
- Jensen K, Pena MG, Wilson RL, Ranganathan UD, Jacobs WR Jr, Fennelly G, Larsen M, Van Rompay KK, Kozlowski PA, Abel K. 2013. A neonatal oral-SIV prime/intramuscular MVA-SIV boost combination vaccine induces both SIV and -specific immune responses in infant macaques. Trials Vaccinol 2:53–63. doi:10.1016/j.trivac.2013.09.005. - DOI - PMC - PubMed
-
- Jensen K, Ranganathan UD, Van Rompay KK, Canfield DR, Khan I, Ravindran R, Luciw PA, Jacobs WR Jr, Fennelly G, Larsen MH, Abel K. 2012. A recombinant attenuated Mycobacterium tuberculosis vaccine strain is safe in immunosuppressed simian immunodeficiency virus-infected infant macaques. Clin Vaccine Immunol 19:1170–1181. doi:10.1128/CVI.00184-12. - DOI - PMC - PubMed
-
- Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, Earl P, Harvey D, Franchini G, Tartaglia J, Montefiori D, Hattangadi S, Moss B, Marthas ML. 2005. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquir Immune Defic Syndr 38:124–134. doi:10.1097/00126334-200502010-00002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F30 AA024374/AA/NIAAA NIH HHS/United States
- P51 OD011107/OD/NIH HHS/United States
- P51 RR000169/RR/NCRR NIH HHS/United States
- HHSN261200800001C/CA/NCI NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- T32 AI007001/AI/NIAID NIH HHS/United States
- T32 AI007419/AI/NIAID NIH HHS/United States
- R24 RR016038/RR/NCRR NIH HHS/United States
- R37 AI026170/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- R01 DE019064/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

