Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma
- PMID: 27252174
- PMCID: PMC6707068
- DOI: 10.1126/scitranslmed.aad9784
Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma
Abstract
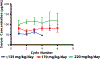
Toca 511 (vocimagene amiretrorepvec) is an investigational nonlytic, retroviral replicating vector (RRV) that delivers a yeast cytosine deaminase, which converts subsequently administered courses of the investigational prodrug Toca FC (extended-release 5-fluorocytosine) into the antimetabolite 5-fluorouracil. Forty-five subjects with recurrent or progressive high-grade glioma were treated. The end points of this phase 1, open-label, ascending dose, multicenter trial included safety, efficacy, and molecular profiling; survival was compared to a matching subgroup from an external control. Overall survival for recurrent high-grade glioma was 13.6 months (95% confidence interval, 10.8 to 20.0) and was statistically improved relative to an external control (hazard ratio, 0.45; P = 0.003). Tumor samples from subjects surviving more than 52 weeks after Toca 511 delivery disproportionately displayed a survival-related mRNA expression signature, identifying a potential molecular signature that may correlate with treatment-related survival rather than being prognostic. Toca 511 and Toca FC show excellent tolerability, with RRV persisting in the tumor and RRV control systemically. The favorable assessment of Toca 511 and Toca FC supports confirmation in a randomized phase 2/3 trial (NCT02414165).
Copyright © 2016, American Association for the Advancement of Science.
Figures


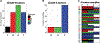
Comment in
-
"Tag Team" Glioblastoma Therapy: Results From a Phase 1 Trial of Toca 511 and 5-Fluorocytosine for Recurrent High-Grade Glioma.Neurosurgery. 2016 Dec;79(6):N18-N20. doi: 10.1227/01.neu.0000508605.38694.fd. Neurosurgery. 2016. PMID: 27861411 No abstract available.
Similar articles
-
Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model.Hum Gene Ther. 2015 Feb;26(2):82-93. doi: 10.1089/hum.2014.100. Epub 2015 Jan 19. Hum Gene Ther. 2015. PMID: 25419577 Free PMC article.
-
Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC.Neuro Oncol. 2018 Sep 3;20(10):1383-1392. doi: 10.1093/neuonc/noy075. Neuro Oncol. 2018. PMID: 29762717 Free PMC article. Clinical Trial.
-
Molecular Analyses Support the Safety and Activity of Retroviral Replicating Vector Toca 511 in Patients.Clin Cancer Res. 2018 Oct 1;24(19):4680-4693. doi: 10.1158/1078-0432.CCR-18-0619. Epub 2018 Jun 26. Clin Cancer Res. 2018. PMID: 29945998 Clinical Trial.
-
Early clinical trials of Toca 511 and Toca FC show a promising novel treatment for recurrent malignant glioma.Expert Opin Investig Drugs. 2019 Mar;28(3):207-216. doi: 10.1080/13543784.2019.1572112. Expert Opin Investig Drugs. 2019. PMID: 30676111 Review.
-
Clinical development of retroviral replicating vector Toca 511 for gene therapy of cancer.Expert Opin Biol Ther. 2021 Sep;21(9):1199-1214. doi: 10.1080/14712598.2021.1902982. Epub 2021 May 6. Expert Opin Biol Ther. 2021. PMID: 33724117 Free PMC article. Review.
Cited by
-
Objective response rate targets for recurrent glioblastoma clinical trials based on the historic association between objective response rate and median overall survival.Neuro Oncol. 2023 Jun 2;25(6):1017-1028. doi: 10.1093/neuonc/noad002. Neuro Oncol. 2023. PMID: 36617262 Free PMC article.
-
Horseradish Peroxidase-Functionalized Gold Nanoconjugates for Breast Cancer Treatment Based on Enzyme Prodrug Therapy.Int J Nanomedicine. 2022 Jan 26;17:409-422. doi: 10.2147/IJN.S323802. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35115775 Free PMC article.
-
How We Treat Recurrent Glioblastoma Today and Current Evidence.Curr Oncol Rep. 2019 Oct 12;21(10):94. doi: 10.1007/s11912-019-0834-y. Curr Oncol Rep. 2019. PMID: 31606796 Review.
-
Benchmarking of Scale-X Bioreactor System in Lentiviral and Adenoviral Vector Production.Hum Gene Ther. 2020 Mar;31(5-6):376-384. doi: 10.1089/hum.2019.247. Hum Gene Ther. 2020. PMID: 32075423 Free PMC article.
-
Current approaches in glioblastoma multiforme immunotherapy.Clin Transl Oncol. 2024 Jul;26(7):1584-1612. doi: 10.1007/s12094-024-03395-7. Epub 2024 Mar 21. Clin Transl Oncol. 2024. PMID: 38512448 Review.
References
-
- Chamberlain MC, Treatment options for glioblastoma. Neurosurgical focus 20, E19 (2006). - PubMed
-
- Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ, Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15, 943–953 (2014). - PubMed
-
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T, Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 27, 4733–4740 (2009). - PubMed
-
- Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, Croteau D, Pedain C, Leland P, Husain SR, Joshi BH, Puri RK, Group PS, Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-oncology 12, 871–881 (2010). - PMC - PubMed
-
- Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S, Ashby LS, Degroot J, Gattamaneni R, Cher L, Rosenthal M, Payer F, Jurgensmeier JM, Jain RK, Sorensen AG, Xu J, Liu Q, van den Bent M, Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 31, 3212–3218 (2013). - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

