Developmental Regulation of the Growth Plate and Cranial Synchondrosis
- PMID: 27250655
- PMCID: PMC5076755
- DOI: 10.1177/0022034516651823
Developmental Regulation of the Growth Plate and Cranial Synchondrosis
Abstract
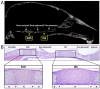
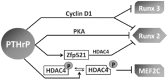
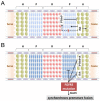
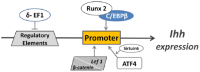
Long bones and the cranial base are both formed through endochondral ossification. Elongation of long bones is primarily through the growth plate, which is a cartilaginous structure at the end of long bones made up of chondrocytes. Growth plate chondrocytes are organized in columns along the longitudinal axis of bone growth. The cranial base is the growth center of the neurocranium. Synchondroses, consisting of mirror-image growth plates, are critical for cranial base elongation and development. Over the last decade, considerable progress has been made in determining the roles of the parathyroid hormone-related protein, Indian hedgehog, fibroblast growth factor, bone morphogenetic protein, and Wnt signaling pathways in various aspects of skeletal development. Furthermore, recent evidence indicates the important role of the primary cilia signaling pathway in bone elongation. Here, we review the development of the growth plate and cranial synchondrosis and the regulation by the above-mentioned signaling pathways, highlighting the similarities and differences between these 2 structures.
Keywords: FGF; Ihh; PTHrP; Wnt; chondrocyte; primary cilia.
© International & American Associations for Dental Research 2016.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures

Similar articles
-
Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function.Dev Biol. 2006 Nov 1;299(1):272-82. doi: 10.1016/j.ydbio.2006.07.028. Epub 2006 Jul 29. Dev Biol. 2006. PMID: 16935278
-
New Insights Into Cranial Synchondrosis Development: A Mini Review.Front Cell Dev Biol. 2020 Aug 11;8:706. doi: 10.3389/fcell.2020.00706. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850826 Free PMC article. Review.
-
Cranial Base Synchondrosis: Chondrocytes at the Hub.Int J Mol Sci. 2022 Jul 15;23(14):7817. doi: 10.3390/ijms23147817. Int J Mol Sci. 2022. PMID: 35887171 Free PMC article. Review.
-
Evc works in chondrocytes and osteoblasts to regulate multiple aspects of growth plate development in the appendicular skeleton and cranial base.Bone. 2012 Jan;50(1):28-41. doi: 10.1016/j.bone.2011.08.025. Epub 2011 Aug 31. Bone. 2012. PMID: 21911092
-
Wnt/beta-catenin signaling regulates cranial base development and growth.J Dent Res. 2008 Mar;87(3):244-9. doi: 10.1177/154405910808700309. J Dent Res. 2008. PMID: 18296608
Cited by
-
Allometry and advancing age significantly structure craniofacial variation in adult female baboons.J Anat. 2019 Aug;235(2):217-232. doi: 10.1111/joa.13005. Epub 2019 May 9. J Anat. 2019. PMID: 31070786 Free PMC article.
-
Sphenoid Bone Determines the Curvature of the Cranial Vault in Postnatal Skull Development in C57BL/6 Mice.J Bone Metab. 2023 Feb;30(1):93-101. doi: 10.11005/jbm.2023.30.1.93. Epub 2023 Feb 28. J Bone Metab. 2023. PMID: 36950845 Free PMC article.
-
Bromodomain and Extra-terminal (BET) Protein Inhibitors Suppress Chondrocyte Differentiation and Restrain Bone Growth.J Biol Chem. 2016 Dec 23;291(52):26647-26657. doi: 10.1074/jbc.M116.749697. Epub 2016 Nov 7. J Biol Chem. 2016. PMID: 27821592 Free PMC article.
-
Selection for increased tibia length in mice alters skull shape through parallel changes in developmental mechanisms.Elife. 2021 Apr 26;10:e67612. doi: 10.7554/eLife.67612. Elife. 2021. PMID: 33899741 Free PMC article.
-
Role of thyroid hormones in craniofacial development.Nat Rev Endocrinol. 2020 Mar;16(3):147-164. doi: 10.1038/s41574-019-0304-5. Epub 2020 Jan 23. Nat Rev Endocrinol. 2020. PMID: 31974498 Review.
References
-
- Caparrós-Martín JA, Valencia M, Reytor E, Pacheco M, Fernandez M, Perez-Aytes A, Gean E, Lapunzina P, Peters H, Goodship JA, et al. 2013. The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia. Hum Mol Genet. 22(1):124–139. - PubMed
-
- Chen L, Li C, Qiao W, Xu X, Deng C. 2001. A Ser(365)–>Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia. Hum Mol Genet. 10(5):457–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

