Current status of non-viral gene therapy for CNS disorders
- PMID: 27249310
- PMCID: PMC5480312
- DOI: 10.1080/17425247.2016.1188802
Current status of non-viral gene therapy for CNS disorders
Abstract
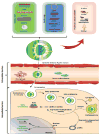
Introduction: Viral and non-viral vectors have been used as methods of delivery in gene therapy for many CNS diseases. Currently, viral vectors such as adeno-associated viruses (AAV), retroviruses, lentiviruses, adenoviruses and herpes simplex viruses (HHV) are being used as successful vectors in gene therapy at clinical trial levels. However, many disadvantages have risen from their usage. Non-viral vectors like cationic polymers, cationic lipids, engineered polymers, nanoparticles, and naked DNA offer a much safer option and can therefore be explored for therapeutic purposes.
Areas covered: This review discusses different types of viral and non-viral vectors for gene therapy and explores clinical trials for CNS diseases that have used these types of vectors for gene delivery. Highlights include non-viral gene delivery and its challenges, possible strategies to improve transfection, regulatory issues concerning vector usage, and future prospects for clinical applications.
Expert opinion: Transfection efficiency of cationic lipids and polymers can be improved through manipulation of molecules used. Efficacy of cationic lipids is dependent on cationic charge, saturation levels, and stability of linkers. Factors determining efficacy of cationic polymers are total charge density, molecular weights, and complexity of molecule. All of the above mentioned parameters must be taken care for efficient gene delivery.
Keywords: Gene delivery; brain delivery; central nervous system; nanobiotechnology; neurological disorders; viral/non-viral vector.
Figures


Similar articles
-
Aerosol gene delivery using viral vectors and cationic carriers for in vivo lung cancer therapy.Expert Opin Drug Deliv. 2015 Jun;12(6):977-91. doi: 10.1517/17425247.2015.986454. Epub 2014 Nov 25. Expert Opin Drug Deliv. 2015. PMID: 25423167 Review.
-
[Advances in cationic polymers used as nonviral vectors for gene delivery].Sheng Wu Gong Cheng Xue Bao. 2013 May;29(5):568-77. Sheng Wu Gong Cheng Xue Bao. 2013. PMID: 24010355 Review. Chinese.
-
Cationic lipid vectors for plasmid DNA delivery.Curr Med Chem. 2003 Jul;10(14):1185-93. doi: 10.2174/0929867033457412. Curr Med Chem. 2003. PMID: 12678793 Review.
-
Recent patents in cationic lipid carriers for delivery of nucleic acids.Recent Pat DNA Gene Seq. 2011 Apr;5(1):8-27. doi: 10.2174/187221511794839255. Recent Pat DNA Gene Seq. 2011. PMID: 21288191 Review.
-
Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy.Eur J Pharm Biopharm. 2004 Jan;57(1):1-8. doi: 10.1016/s0939-6411(03)00155-3. Eur J Pharm Biopharm. 2004. PMID: 14729076 Review.
Cited by
-
DNA nuclear targeting sequences for enhanced non-viral gene transfer: An in vitro and in vivo study.Mol Ther Nucleic Acids. 2021 Mar 19;24:477-486. doi: 10.1016/j.omtn.2021.03.012. eCollection 2021 Jun 4. Mol Ther Nucleic Acids. 2021. PMID: 33898102 Free PMC article.
-
Nanosystems for gene therapy targeting brain damage caused by viral infections.Mater Today Bio. 2022 Dec 17;18:100525. doi: 10.1016/j.mtbio.2022.100525. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36619201 Free PMC article. Review.
-
Nanocarriers for Delivery of Oligonucleotides to the CNS.Int J Mol Sci. 2022 Jan 11;23(2):760. doi: 10.3390/ijms23020760. Int J Mol Sci. 2022. PMID: 35054957 Free PMC article. Review.
-
Effects of gene therapy on cardiovascular symptoms of lysosomal storage diseases.Genet Mol Biol. 2019;42(1 suppl 1):261-285. doi: 10.1590/1678-4685-GMB-2018-0100. Epub 2019 May 23. Genet Mol Biol. 2019. PMID: 31132295 Free PMC article.
-
Advancements in nano-enabled therapeutics for neuroHIV management.Int J Nanomedicine. 2016 Sep 1;11:4317-25. doi: 10.2147/IJN.S109943. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27621624 Free PMC article.
References
-
- Wang W, Li W, Ma N, et al. Non-viral gene delivery methods. Curr Pharm Biotechnol. 2013;14(1):46–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
