Oncogenic mutations in the FBXW7 gene of adult T-cell leukemia patients
- PMID: 27247421
- PMCID: PMC4914202
- DOI: 10.1073/pnas.1601537113
Oncogenic mutations in the FBXW7 gene of adult T-cell leukemia patients
Abstract
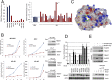
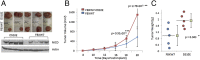
Human T-cell leukemia virus type 1 (HTLV-I) is associated with adult T-cell leukemia (ATL), an aggressive lymphoproliferative disease with a dismal prognosis. We have previously described the presence of Notch1 activating mutations and constitutive Notch1 signaling in patients with acute ATL. In this study, we report a high frequency of F-box and WD repeat domain containing 7 (FBXW7)/hCDC4 mutations within the WD40 substrate-binding domain in 8 of 32 acute ATL patients (25%). Functionally, ATL FBXW7 mutants lost their ability to interact with intracellular Notch (NICD), resulting in increased protein stability and constitutive Notch1 signaling. Consistent with the loss-of-function found in ATL patients, expression of WT FBXW7 in several patient-derived ATL lines demonstrated strong tumor-suppressor activity characterized by reduced proliferation of ATL cells. Remarkably, two FBXW7 mutants, D510E and D527G, demonstrated oncogenic activity when expressed in the presence of HTLV-I Tax, mutated p53 R276H, or c-Myc F138C found in human cancers. Transforming activity was further demonstrated by the ability of the FBXW7 D510E mutant to provide IL-2-independent growth of Tax-immortalized human T cells and increase the tumor formation in a xenograft mouse model of ATL. This study suggests that FBXW7, normally a tumor suppressor, can act as an oncogene when mutated and may play an important role in the pathogenesis of ATL.
Keywords: FBXW7; HTLV; Notch; leukemia; oncogene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Loss of FBXW7-mediated degradation of BRAF elicits resistance to BET inhibitors in adult T cell leukemia cells.Mol Cancer. 2020 Sep 9;19(1):139. doi: 10.1186/s12943-020-01254-x. Mol Cancer. 2020. PMID: 32907612 Free PMC article.
-
Notch signaling contributes to proliferation and tumor formation of human T-cell leukemia virus type 1-associated adult T-cell leukemia.Proc Natl Acad Sci U S A. 2010 Sep 21;107(38):16619-24. doi: 10.1073/pnas.1010722107. Epub 2010 Sep 7. Proc Natl Acad Sci U S A. 2010. PMID: 20823234 Free PMC article.
-
The tumor suppressor gene hCDC4 is frequently mutated in human T-cell acute lymphoblastic leukemia with functional consequences for Notch signaling.Cancer Res. 2007 Jun 15;67(12):5611-6. doi: 10.1158/0008-5472.CAN-06-4381. Cancer Res. 2007. PMID: 17575125
-
Regulation mechanism of Fbxw7-related signaling pathways (Review).Oncol Rep. 2015 Nov;34(5):2215-24. doi: 10.3892/or.2015.4227. Epub 2015 Aug 26. Oncol Rep. 2015. PMID: 26324296 Review.
-
Therapeutic targeting of NOTCH1 signaling in T-cell acute lymphoblastic leukemia.Clin Lymphoma Myeloma. 2009;9 Suppl 3(Suppl 3):S205-10. doi: 10.3816/CLM.2009.s.013. Clin Lymphoma Myeloma. 2009. PMID: 19778842 Free PMC article. Review.
Cited by
-
Decoding the PTM-switchboard of Notch.Biochim Biophys Acta Mol Cell Res. 2019 Dec;1866(12):118507. doi: 10.1016/j.bbamcr.2019.07.002. Epub 2019 Jul 11. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31301363 Free PMC article. Review.
-
Biology and Molecular Pathogenesis of Mature T-Cell Lymphomas.Cold Spring Harb Perspect Med. 2021 May 3;11(5):a035402. doi: 10.1101/cshperspect.a035402. Cold Spring Harb Perspect Med. 2021. PMID: 32513675 Free PMC article. Review.
-
Loss of FBXW7-mediated degradation of BRAF elicits resistance to BET inhibitors in adult T cell leukemia cells.Mol Cancer. 2020 Sep 9;19(1):139. doi: 10.1186/s12943-020-01254-x. Mol Cancer. 2020. PMID: 32907612 Free PMC article.
-
Emerging roles of the HECT-type E3 ubiquitin ligases in hematological malignancies.Discov Oncol. 2021 Oct 8;12(1):39. doi: 10.1007/s12672-021-00435-4. Discov Oncol. 2021. PMID: 35201500 Free PMC article. Review.
-
Comparison of Clinical Characteristics and Genetic Aberrations of Plasma Cell Disorders in Thailand Population.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221111228. doi: 10.1177/15330338221111228. Technol Cancer Res Treat. 2022. PMID: 35770320 Free PMC article.
References
-
- Sun SC, Yamaoka S. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene. 2005;24(39):5952–5964. - PubMed
-
- Sinha-Datta U, et al. Transcriptional activation of hTERT through the NF-kappaB pathway in HTLV-I-transformed cells. Blood. 2004;104(8):2523–2531. - PubMed
-
- Tabakin-Fix Y, Azran I, Schavinky-Khrapunsky Y, Levy O, Aboud M. Functional inactivation of p53 by human T-cell leukemia virus type 1 Tax protein: mechanisms and clinical implications. Carcinogenesis. 2006;27(4):673–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

