IgG subclasses determine pathways of anaphylaxis in mice
- PMID: 27246523
- PMCID: PMC5081282
- DOI: 10.1016/j.jaci.2016.03.028
IgG subclasses determine pathways of anaphylaxis in mice
Abstract
Background: Animal models have demonstrated that allergen-specific IgG confers sensitivity to systemic anaphylaxis that relies on IgG Fc receptors (FcγRs). Mouse IgG2a and IgG2b bind activating FcγRI, FcγRIII, and FcγRIV and inhibitory FcγRIIB; mouse IgG1 binds only FcγRIII and FcγRIIB. Although these interactions are of strikingly different affinities, these 3 IgG subclasses have been shown to enable induction of systemic anaphylaxis.
Objective: We sought to determine which pathways control the induction of IgG1-, IgG2a-, and IgG2b-dependent passive systemic anaphylaxis.
Methods: Mice were sensitized with IgG1, IgG2a, or IgG2b anti-trinitrophenyl mAbs and challenged with trinitrophenyl-BSA intravenously to induce systemic anaphylaxis that was monitored by using rectal temperature. Anaphylaxis was evaluated in mice deficient for FcγRs injected with mediator antagonists or in which basophils, monocytes/macrophages, or neutrophils had been depleted. FcγR expression was evaluated on these cells before and after anaphylaxis.
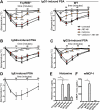
Results: Activating FcγRIII is the receptor primarily responsible for all 3 models of anaphylaxis, and subsequent downregulation of this receptor was observed. These models differentially relied on histamine release and the contribution of mast cells, basophils, macrophages, and neutrophils. Strikingly, basophil contribution and histamine predominance in mice with IgG1- and IgG2b-induced anaphylaxis correlated with the ability of inhibitory FcγRIIB to negatively regulate these models of anaphylaxis.
Conclusion: We propose that the differential expression of inhibitory FcγRIIB on myeloid cells and its differential binding of IgG subclasses controls the contributions of mast cells, basophils, neutrophils, and macrophages to IgG subclass-dependent anaphylaxis. Collectively, our results unravel novel complexities in the involvement and regulation of cell populations in IgG-dependent reactions in vivo.
Keywords: Anaphylaxis; IgG; IgG Fc receptor; basophil; histamine; macrophage; monocyte; mouse model; neutrophil; platelet-activating factor.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
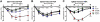
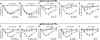



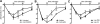
Similar articles
-
Rapid desensitization of mice with anti-FcγRIIb/FcγRIII mAb safely prevents IgG-mediated anaphylaxis.J Allergy Clin Immunol. 2013 Dec;132(6):1375-87. doi: 10.1016/j.jaci.2013.09.008. Epub 2013 Oct 18. J Allergy Clin Immunol. 2013. PMID: 24139828 Free PMC article.
-
Anti-FcγRIIB mAb suppresses murine IgG-dependent anaphylaxis by Fc domain targeting of FcγRIII.J Allergy Clin Immunol. 2018 Apr;141(4):1373-1381.e5. doi: 10.1016/j.jaci.2017.05.027. Epub 2017 Jun 15. J Allergy Clin Immunol. 2018. PMID: 28624610 Free PMC article.
-
FcgammaRIV: a novel FcR with distinct IgG subclass specificity.Immunity. 2005 Jul;23(1):41-51. doi: 10.1016/j.immuni.2005.05.010. Immunity. 2005. PMID: 16039578
-
Anaphylaxis: lessons from mouse models.J Allergy Clin Immunol. 2007 Sep;120(3):506-15; quiz 516-7. doi: 10.1016/j.jaci.2007.07.033. J Allergy Clin Immunol. 2007. PMID: 17765751 Review.
-
Human IgE-independent systemic anaphylaxis.J Allergy Clin Immunol. 2016 Jun;137(6):1674-1680. doi: 10.1016/j.jaci.2016.02.015. Epub 2016 Apr 26. J Allergy Clin Immunol. 2016. PMID: 27130857 Free PMC article. Review.
Cited by
-
Anaphylaxis: Focus on Transcription Factor Activity.Int J Mol Sci. 2021 May 6;22(9):4935. doi: 10.3390/ijms22094935. Int J Mol Sci. 2021. PMID: 34066544 Free PMC article. Review.
-
Mimicking Antigen-Driven Asthma in Rodent Models-How Close Can We Get?Front Immunol. 2020 Sep 30;11:575936. doi: 10.3389/fimmu.2020.575936. eCollection 2020. Front Immunol. 2020. PMID: 33101301 Free PMC article. Review.
-
Obesity affects peripheral lymphoid organs immune response in murine asthma model.Immunology. 2019 Jul;157(3):268-279. doi: 10.1111/imm.13081. Immunology. 2019. PMID: 31112301 Free PMC article.
-
Mouse Models and Tools for the in vivo Study of Neutrophils.Front Immunol. 2020 Jan 21;10:3130. doi: 10.3389/fimmu.2019.03130. eCollection 2019. Front Immunol. 2020. PMID: 32038641 Free PMC article. Review.
-
Alternative Anaphylactic Routes: The Potential Role of Macrophages.Front Immunol. 2017 May 8;8:515. doi: 10.3389/fimmu.2017.00515. eCollection 2017. Front Immunol. 2017. PMID: 28533777 Free PMC article. Review.
References
-
- Brown SG, Stone SF, Fatovich DM, Burrows SA, Holdgate A, Celenza A, et al. Anaphylaxis: Clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013 - PubMed
-
- Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–57. quiz 58. - PubMed
-
- Jonsson F, Mancardi DA, Albanesi M, Bruhns P. Neutrophils in local and systemic antibody-dependent inflammatory and anaphylactic reactions. J Leukoc Biol. 2013;94:643–56. - PubMed
-
- Iff ET, Vaz NM. Mechanisms of anaphylaxis in the mouse. Similarity of shock induced by anaphylaxis and by mixtures of histamine and serotonin. Int Arch Allergy Appl Immunol. 1966;30:313–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

