Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis
- PMID: 27245276
- PMCID: PMC4956571
- DOI: 10.1016/j.lfs.2016.05.036
Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis
Abstract
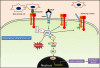
The Src-family kinases (SFKs), an intracellularly located group of non-receptor tyrosine kinases are involved in oncogenesis. The importance of SFKs has been implicated in the promotion of tumor cell motility, proliferation, inhibition of apoptosis, invasion and metastasis. Recent evidences indicate that specific effects of SFKs on epithelial-to-mesenchymal transition (EMT) as well as on endothelial and stromal cells in the tumor microenvironment can have profound effects on tumor microinvasion and metastasis. Although, having been studied extensively, these novel features of SFKs may contribute to greater understanding of benefits from Src inhibition in various types of cancers. Here we review the novel role of SFKs, particularly c-Src in mediating EMT, modulation of tumor endothelial-barrier, transendothelial migration (microinvasion) and metastasis of cancer cells, and discuss the utility of Src inhibitors in vascular normalization and cancer therapy.
Keywords: EMT; Metastasis; Microinvasion; Src; Vascular permeability.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

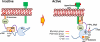
Similar articles
-
Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis.Cell Tissue Res. 2009 Jan;335(1):249-59. doi: 10.1007/s00441-008-0682-9. Epub 2008 Sep 25. Cell Tissue Res. 2009. PMID: 18815812 Free PMC article. Review.
-
c-Src activation promotes nasopharyngeal carcinoma metastasis by inducing the epithelial-mesenchymal transition via PI3K/Akt signaling pathway: a new and promising target for NPC.Oncotarget. 2016 May 10;7(19):28340-55. doi: 10.18632/oncotarget.8634. Oncotarget. 2016. PMID: 27078847 Free PMC article.
-
Src, chemoresistance and epithelial to mesenchymal transition: are they related?Anticancer Drugs. 2007 Apr;18(4):371-5. doi: 10.1097/CAD.0b013e32801265d7. Anticancer Drugs. 2007. PMID: 17351389 Review.
-
Differential roles of Src in transforming growth factor-ß regulation of growth arrest, epithelial-to-mesenchymal transition and cell migration in pancreatic ductal adenocarcinoma cells.Int J Oncol. 2011 Mar;38(3):797-805. doi: 10.3892/ijo.2011.897. Epub 2011 Jan 11. Int J Oncol. 2011. PMID: 21225226
-
The Leptin induced Hic-5 expression and actin puncta formation by the FAK/Src-dependent pathway in MCF10A mammary epithelial cells.Biomedica. 2019 Sep 1;39(3):547-560. doi: 10.7705/biomedica.4313. Biomedica. 2019. PMID: 31584768 Free PMC article. English, Spanish.
Cited by
-
Src Inhibitors Pyrazolo[3,4-d]pyrimidines, Si306 and Pro-Si306, Inhibit Focal Adhesion Kinase and Suppress Human Glioblastoma Invasion In Vitro and In Vivo.Cancers (Basel). 2020 Jun 14;12(6):1570. doi: 10.3390/cancers12061570. Cancers (Basel). 2020. PMID: 32545852 Free PMC article.
-
Ca2+ Signaling and Src Functions in Tumor Cells.Biomolecules. 2023 Dec 3;13(12):1739. doi: 10.3390/biom13121739. Biomolecules. 2023. PMID: 38136610 Free PMC article. Review.
-
MAPK Reliance via Acquired CDK4/6 Inhibitor Resistance in Cancer.Clin Cancer Res. 2018 Sep 1;24(17):4201-4214. doi: 10.1158/1078-0432.CCR-18-0410. Epub 2018 May 8. Clin Cancer Res. 2018. PMID: 29739788 Free PMC article.
-
Clinical efficacy and potential mechanism of ginseng polysaccharides in the treatment of non-small cell lung cancer based on meta-analysis associated with network pharmacology.Heliyon. 2024 Mar 7;10(6):e27152. doi: 10.1016/j.heliyon.2024.e27152. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38496882 Free PMC article.
-
Vascular Normalization: A New Window Opened for Cancer Therapies.Front Oncol. 2021 Aug 12;11:719836. doi: 10.3389/fonc.2021.719836. eCollection 2021. Front Oncol. 2021. PMID: 34476218 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

