ALG-2 interacting protein-X (Alix) is essential for clathrin-independent endocytosis and signaling
- PMID: 27244115
- PMCID: PMC4886688
- DOI: 10.1038/srep26986
ALG-2 interacting protein-X (Alix) is essential for clathrin-independent endocytosis and signaling
Abstract
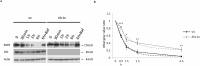
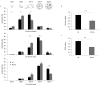
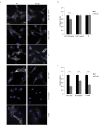
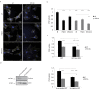
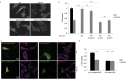
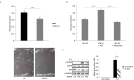
The molecular mechanisms and the biological functions of clathrin independent endocytosis (CIE) remain largely elusive. Alix (ALG-2 interacting protein X), has been assigned roles in membrane deformation and fission both in endosomes and at the plasma membrane. Using Alix ko cells, we show for the first time that Alix regulates fluid phase endocytosis and internalization of cargoes entering cells via CIE, but has no apparent effect on clathrin mediated endocytosis or downstream endosomal trafficking. We show that Alix acts with endophilin-A to promote CIE of cholera toxin and to regulate cell migration. We also found that Alix is required for fast endocytosis and downstream signaling of the interleukin-2 receptor giving a first indication that CIE is necessary for activation of at least some surface receptors. In addition to characterizing a new function for Alix, our results highlight Alix ko cells as a unique tool to unravel the biological consequences of CIE.
Figures

Similar articles
-
Alix regulates cortical actin and the spatial distribution of endosomes.J Cell Sci. 2005 Jun 15;118(Pt 12):2625-35. doi: 10.1242/jcs.02382. Epub 2005 May 24. J Cell Sci. 2005. PMID: 15914539
-
Alix and ALG-2 are involved in tumor necrosis factor receptor 1-induced cell death.J Biol Chem. 2008 Dec 12;283(50):34954-65. doi: 10.1074/jbc.M803140200. Epub 2008 Oct 20. J Biol Chem. 2008. PMID: 18936101 Free PMC article.
-
Interaction of HIV-1 Nef protein with the host protein Alix promotes lysosomal targeting of CD4 receptor.J Biol Chem. 2014 Oct 3;289(40):27744-56. doi: 10.1074/jbc.M114.560193. Epub 2014 Aug 12. J Biol Chem. 2014. PMID: 25118280 Free PMC article.
-
Alix and ALG-2 make a link between endosomes and neuronal death.Biochem Soc Trans. 2009 Feb;37(Pt 1):200-3. doi: 10.1042/BST0370200. Biochem Soc Trans. 2009. PMID: 19143631 Review.
-
Do Alix and ALG-2 really control endosomes for better or for worse?Biol Cell. 2006 Jan;98(1):69-77. doi: 10.1042/BC20050007. Biol Cell. 2006. PMID: 16354163 Review.
Cited by
-
The Ins and Outs of Antigen Uptake in B cells.Front Immunol. 2022 Apr 26;13:892169. doi: 10.3389/fimmu.2022.892169. eCollection 2022. Front Immunol. 2022. PMID: 35572544 Free PMC article. Review.
-
Endophilin A2 regulates B-cell endocytosis and is required for germinal center and humoral responses.EMBO Rep. 2021 Sep 6;22(9):e51328. doi: 10.15252/embr.202051328. Epub 2021 Jul 29. EMBO Rep. 2021. PMID: 34323351 Free PMC article.
-
Multifaceted Roles of ALG-2 in Ca(2+)-Regulated Membrane Trafficking.Int J Mol Sci. 2016 Aug 26;17(9):1401. doi: 10.3390/ijms17091401. Int J Mol Sci. 2016. PMID: 27571067 Free PMC article. Review.
-
Alix: A Candidate Serum Biomarker of Alzheimer's Disease.Front Aging Neurosci. 2021 Jun 15;13:669612. doi: 10.3389/fnagi.2021.669612. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34211388 Free PMC article.
-
Cholera Toxin as a Probe for Membrane Biology.Toxins (Basel). 2021 Aug 3;13(8):543. doi: 10.3390/toxins13080543. Toxins (Basel). 2021. PMID: 34437414 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

