Multi-Compartmentalisation in the MAPK Signalling Pathway Contributes to the Emergence of Oscillatory Behaviour and to Ultrasensitivity
- PMID: 27243235
- PMCID: PMC4887093
- DOI: 10.1371/journal.pone.0156139
Multi-Compartmentalisation in the MAPK Signalling Pathway Contributes to the Emergence of Oscillatory Behaviour and to Ultrasensitivity
Abstract
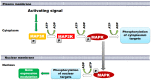
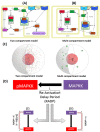
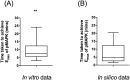
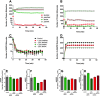
Signal transduction through the Mitogen Activated Protein Kinase (MAPK) pathways is evolutionarily highly conserved. Many cells use these pathways to interpret changes to their environment and respond accordingly. The pathways are central to triggering diverse cellular responses such as survival, apoptosis, differentiation and proliferation. Though the interactions between the different MAPK pathways are complex, nevertheless, they maintain a high level of fidelity and specificity to the original signal. There are numerous theories explaining how fidelity and specificity arise within this complex context; spatio-temporal regulation of the pathways and feedback loops are thought to be very important. This paper presents an agent based computational model addressing multi-compartmentalisation and how this influences the dynamics of MAPK cascade activation. The model suggests that multi-compartmentalisation coupled with periodic MAPK kinase (MAPKK) activation may be critical factors for the emergence of oscillation and ultrasensitivity in the system. Finally, the model also establishes a link between the spatial arrangements of the cascade components and temporal activation mechanisms, and how both contribute to fidelity and specificity of MAPK mediated signalling.
Conflict of interest statement
Figures
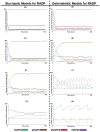
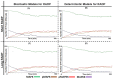
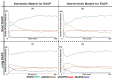
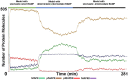
Similar articles
-
Random parameter sampling of a generic three-tier MAPK cascade model reveals major factors affecting its versatile dynamics.PLoS One. 2013;8(1):e54441. doi: 10.1371/journal.pone.0054441. Epub 2013 Jan 24. PLoS One. 2013. PMID: 23365667 Free PMC article.
-
Integration of Caenorhabditis elegans MAPK pathways mediating immunity and stress resistance by MEK-1 MAPK kinase and VHP-1 MAPK phosphatase.Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):10990-4. doi: 10.1073/pnas.0403546101. Epub 2004 Jul 15. Proc Natl Acad Sci U S A. 2004. PMID: 15256594 Free PMC article.
-
A docking site determining specificity of Pbs2 MAPKK for Ssk2/Ssk22 MAPKKKs in the yeast HOG pathway.EMBO J. 2003 Jul 15;22(14):3624-34. doi: 10.1093/emboj/cdg353. EMBO J. 2003. PMID: 12853477 Free PMC article.
-
Scaffold proteins in mammalian MAP kinase cascades.J Biochem. 2004 Jun;135(6):657-61. doi: 10.1093/jb/mvh079. J Biochem. 2004. PMID: 15213240 Review.
-
Regulation of Mitogen-Activated Protein Kinase Signaling Pathways by the Ubiquitin-Proteasome System and Its Pharmacological Potential.Pharmacol Rev. 2021 Oct;73(4):263-296. doi: 10.1124/pharmrev.120.000170. Pharmacol Rev. 2021. PMID: 34732541 Review.
Cited by
-
Connecting Agent-Based Models with High-Dimensional Parameter Spaces to Multidimensional Data Using SMoRe ParS: A Surrogate Modeling Approach.Bull Math Biol. 2023 Dec 30;86(1):11. doi: 10.1007/s11538-023-01240-6. Bull Math Biol. 2023. PMID: 38159216 Free PMC article.
-
Comparative Transcriptome Analysis Reveals Growth-Related Genes in Juvenile Chinese Sea Cucumber, Russian Sea Cucumber, and Their Hybrids.Mar Biotechnol (NY). 2018 Apr;20(2):193-205. doi: 10.1007/s10126-018-9796-6. Epub 2018 Feb 28. Mar Biotechnol (NY). 2018. PMID: 29492749
-
Agent-Based Modeling of Complex Molecular Systems.Methods Mol Biol. 2022;2399:367-391. doi: 10.1007/978-1-0716-1831-8_15. Methods Mol Biol. 2022. PMID: 35604564
-
Analysis of mechanotransduction dynamics during combined mechanical stimulation and modulation of the extracellular-regulated kinase cascade uncovers hidden information within the signalling noise.Interface Focus. 2021 Feb 6;11(1):20190136. doi: 10.1098/rsfs.2019.0136. Epub 2020 Dec 11. Interface Focus. 2021. PMID: 33343875 Free PMC article.
-
Revealing hidden information in osteoblast's mechanotransduction through analysis of time patterns of critical events.BMC Bioinformatics. 2020 Mar 18;21(1):114. doi: 10.1186/s12859-020-3394-0. BMC Bioinformatics. 2020. PMID: 32183690 Free PMC article.
References
-
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1995;9(9):726–35. Epub 1995/06/01. . - PubMed
-
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, et al. Phosphorylation of the MAP Kinase ERK2 Promotes Its Homodimerization and Nuclear Translocation. Cell. 1998;93(4):605–15. - PubMed
-
- Chambard J-C, Lefloch R, Pouysségur J, Lenormand P. ERK implication in cell cycle regulation. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 2007;1773(8):1299–310. - PubMed
-
- Choi TG, Lee J, Ha J, Kim SS. Apoptosis signal-regulating kinase 1 is an intracellular inducer of p38 MAPK-mediated myogenic signalling in cardiac myoblasts. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 2011;1813(8):1412–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

