Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study
- PMID: 27240787
- PMCID: PMC4914044
- DOI: 10.1016/S2352-3018(16)30011-X
Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study
Abstract
Background: For second-line antiretroviral therapy, WHO recommends a boosted protease inhibitor plus nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs). However, concerns about toxicity and cross-resistance motivated a search for regimens that do not contain NRTIs. We aimed to assess whether boosted lopinavir plus raltegravir would be non-inferior to boosted lopinavir plus NRTIs for virological suppression in resource-limited settings.
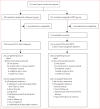
Methods: A5273 was a randomised, open-label, phase 3, non-inferiority study at 15 AIDS Clinical Trials Group (ACTG) research sites in nine resource-limited countries (three sites each in India and South Africa, two each in Malawi and Peru, and one each in Brazil, Kenya, Tanzania, Thailand, and Zimbabwe). Adults with plasma HIV-1 RNA concentrations of at least 1000 copies per mL after at least 24 weeks on a regimen based on a non-NRTI inhibitor were randomly assigned (1:1) to receive oral ritonavir-boosted lopinavir (100 mg ritonavir, 400 mg lopinavir) plus 400 mg raltegravir twice a day (raltegravir group) or to ritonavir-boosted lopinavir plus two or three NRTIs selected from an algorithm (eg, zidovudine after failure with tenofovir and vice versa; NRTI group). Randomised group assignment was done with a computer algorithm concealed to site personnel, and stratified by HIV-1 RNA viral load, CD4 cell count, and intention to use zidovudine, with the groups balanced by each site. The primary endpoint was time to confirmed virological failure (two measurements of HIV-1 RNA viral load >400 copies per mL) at or after week 24 in the intention-to-treat population. Non-inferiority (10% margin) was assessed by comparing the cumulative probability of virological failure by 48 weeks. This trial was registered with ClinicalTrials.gov, NCT01352715.
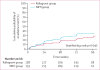
Findings: Between March 13, 2012, and Oct 2, 2013, we randomly assigned 515 participants: 260 to the raltegravir group and 255 to the NRTI group; two participants in the raltegravir group and one in the NRTI group were excluded from analyses because of ineligibility. By the end of follow-up (October, 2014), 96 participants had virological failure (46 in the raltegravir group and 50 in the NRTI group). By 48 weeks, the cumulative probability of virological failure was 10·3% (95% CI 6·5-14·0) in the raltegravir group and 12·4% (8·3-16·5) in the NRTI group, with a weighted difference of -3·4% (-8·4 to 1·5), indicating that raltegravir was non-inferior, but not superior, to NRTIs. 62 (24%) participants in the raltegravir group and 81 (32%) in the NRTI group had grade 3 or higher adverse events; 19 (7%) and 29 (11%), respectively, had serious adverse events. Three participants in each group died, all from HIV-related causes.
Interpretation: In settings with extensive NRTI resistance but no available resistance testing, our data support WHO's recommendation for ritonavir-boosted lopinavir plus NRTI for second-line antiretroviral therapy. Ritonavir-boosted lopinavir plus raltegravir is an appropriate alternative, especially if NRTI use is limited by toxicity.
Funding: National Institutes of Health.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
AMLR has received salary support from Johnson & Johnson (Peru) and Merck Sharp & Dohme (Peru). BT has received research grants and honoraria from ViiV, and honoraria from Gilead Sciences, GlaxoSmithKline, and Janssen. CLW has received honoraria from Abbvie/Abbott, Celera, and Merck Sharp & Dohme. JWM has received funds from Gilead Sciences, support from Cocrystal Pharma, and has a patent for 3’Azido Purine Nucleotide Prodrugs for treatment of viral infections (number 8, 815, 829). ACC has received research support from Bristol-Myers Squibb, Merck Sharp & Dohme, and Roche Molecular Systems; and honoraria from Merck Sharp & Dohme for data and safety monitoring board membership. All other authors declare no competing interests.
Figures


Comment in
-
Raltegravir in second-line ART in resource-limited settings.Lancet HIV. 2016 Jun;3(6):e240-1. doi: 10.1016/S2352-3018(16)30014-5. Epub 2016 Apr 18. Lancet HIV. 2016. PMID: 27240783 No abstract available.
Similar articles
-
Lopinavir plus nucleoside reverse-transcriptase inhibitors, lopinavir plus raltegravir, or lopinavir monotherapy for second-line treatment of HIV (EARNEST): 144-week follow-up results from a randomised controlled trial.Lancet Infect Dis. 2018 Jan;18(1):47-57. doi: 10.1016/S1473-3099(17)30630-8. Epub 2017 Nov 3. Lancet Infect Dis. 2018. PMID: 29108797 Free PMC article. Clinical Trial.
-
Body composition and metabolic outcomes after 96 weeks of treatment with ritonavir-boosted lopinavir plus either nucleoside or nucleotide reverse transcriptase inhibitors or raltegravir in patients with HIV with virological failure of a standard first-line antiretroviral therapy regimen: a substudy of the randomised, open-label, non-inferiority SECOND-LINE study.Lancet HIV. 2017 Jan;4(1):e13-e20. doi: 10.1016/S2352-3018(16)30189-8. Epub 2016 Nov 1. Lancet HIV. 2017. PMID: 27815068 Clinical Trial.
-
Efficacy and safety of three antiretroviral therapy regimens for treatment-naive African adults living with HIV-2 (FIT-2): a pilot, phase 2, non-comparative, open-label, randomised controlled trial.Lancet HIV. 2024 Jun;11(6):e380-e388. doi: 10.1016/S2352-3018(24)00085-7. Epub 2024 May 10. Lancet HIV. 2024. PMID: 38740027 Clinical Trial.
-
Comparative efficacy and safety of second-line antiretroviral therapy for treatment of HIV/AIDS: a systematic review and network meta-analysis.Lancet HIV. 2017 Oct;4(10):e433-e441. doi: 10.1016/S2352-3018(17)30109-1. Epub 2017 Aug 4. Lancet HIV. 2017. PMID: 28784426 Review.
-
Effects of nucleoside reverse transcriptase inhibitor backbone on the efficacy of first-line boosted highly active antiretroviral therapy based on protease inhibitors: meta-regression analysis of 12 clinical trials in 5168 patients.HIV Med. 2009 Oct;10(9):527-35. doi: 10.1111/j.1468-1293.2009.00724.x. HIV Med. 2009. PMID: 19785663 Review.
Cited by
-
Diverse Human Immunodeficiency Virus-1 Drug Resistance Profiles at Screening for ACTG A5288: A Study of People Experiencing Virologic Failure on Second-line Antiretroviral Therapy in Resource-limited Settings.Clin Infect Dis. 2020 Oct 23;71(7):e170-e177. doi: 10.1093/cid/ciz1116. Clin Infect Dis. 2020. PMID: 31724034 Free PMC article.
-
Efficacy and Safety of Raltegravir-Based Dual Therapy in AIDS Patients: A Meta-Analysis of Randomized Controlled Trials.Front Pharmacol. 2019 Oct 17;10:1225. doi: 10.3389/fphar.2019.01225. eCollection 2019. Front Pharmacol. 2019. PMID: 31749699 Free PMC article.
-
Virologic efficacy of tenofovir, lamivudine and dolutegravir as second-line antiretroviral therapy in adults failing a tenofovir-based first-line regimen.AIDS. 2021 Jul 15;35(9):1423-1432. doi: 10.1097/QAD.0000000000002936. AIDS. 2021. PMID: 33973876 Free PMC article.
-
Incidence and Predictors of Pregnancy in Women Enrolled in Large Multinational HIV Treatment Trials of the AIDS Clinical Trials Group.J Acquir Immune Defic Syndr. 2023 Dec 15;94(5):461-467. doi: 10.1097/QAI.0000000000003299. J Acquir Immune Defic Syndr. 2023. PMID: 37820116 Free PMC article.
-
Lopinavir plus nucleoside reverse-transcriptase inhibitors, lopinavir plus raltegravir, or lopinavir monotherapy for second-line treatment of HIV (EARNEST): 144-week follow-up results from a randomised controlled trial.Lancet Infect Dis. 2018 Jan;18(1):47-57. doi: 10.1016/S1473-3099(17)30630-8. Epub 2017 Nov 3. Lancet Infect Dis. 2018. PMID: 29108797 Free PMC article. Clinical Trial.
References
-
- UNAIDS. “15 by 15” A Global Target Achieved. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_15by15_en.pdf (accessed Sept 8, 2015).
-
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 Sep; http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ (accessed Oct 13, 2015). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI069423/AI/NIAID NIH HHS/United States
- U01 AI069438/AI/NIAID NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI108568/AI/NIAID NIH HHS/United States
- UM1 AI069417/AI/NIAID NIH HHS/United States
- UM1 AI069497/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- U01 AI069497/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- U01 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI069438/AI/NIAID NIH HHS/United States
- U01 AI069484/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

