Functions of Prdm16 in thermogenic fat cells
- PMID: 27227007
- PMCID: PMC4843880
- DOI: 10.4161/23328940.2014.974444
Functions of Prdm16 in thermogenic fat cells
Abstract
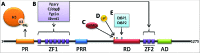
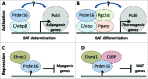
The PR-domain containing 16 (Prdm16) protein is a powerful inducer of the thermogenic phenotype in fat cells. In both developmental (brown) and induced (beige) thermogenic adipose tissue, Prdm16 has a critical role in maintaining proper tissue structure and function. It has roles throughout the course of differentiation, beginning with lineage determination activity in precursor cells, and continuing with coactivator functions that enable and maintain thermogenic gene expression. These abilities are primarily mediated by interactions with other adipogenic factors, suggesting that Prdm16 acts to coordinate the overall brown adipose phenotype. Mouse models have confirmed that thermogenic adipose depends upon Prdm16, and that this type of fat tissue provides substantial metabolic protection against the harmful effects of a high fat/high energy diet. Activation of Prdm16, therefore, holds promise for stimulating thermogenesis in fat cells to reduce human obesity and its complications.
Keywords: BAT; Prdm16; WAT; adipogenesis; beige/brown/white adipose tissue; obesity; thermogenesis.
Figures

Similar articles
-
Functional thermogenic beige adipogenesis is inducible in human neck fat.Int J Obes (Lond). 2014 Feb;38(2):170-6. doi: 10.1038/ijo.2013.82. Epub 2013 May 21. Int J Obes (Lond). 2014. PMID: 23736373 Free PMC article.
-
Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice.J Clin Invest. 2011 Jan;121(1):96-105. doi: 10.1172/JCI44271. Epub 2010 Dec 1. J Clin Invest. 2011. PMID: 21123942 Free PMC article.
-
Role of PRDM16 in the activation of brown fat programming. Relevance to the development of obesity.Histol Histopathol. 2013 Nov;28(11):1411-25. doi: 10.14670/HH-28.1411. Epub 2013 Jun 17. Histol Histopathol. 2013. PMID: 23771475 Review.
-
Wt1 haploinsufficiency induces browning of epididymal fat and alleviates metabolic dysfunction in mice on high-fat diet.Diabetologia. 2022 Mar;65(3):528-540. doi: 10.1007/s00125-021-05621-1. Epub 2021 Nov 30. Diabetologia. 2022. PMID: 34846543 Free PMC article.
-
White, brown, beige/brite: different adipose cells for different functions?Endocrinology. 2013 Sep;154(9):2992-3000. doi: 10.1210/en.2013-1403. Epub 2013 Jun 19. Endocrinology. 2013. PMID: 23782940 Review.
Cited by
-
Bifidobacterium bifidum DS0908 and Bifidobacterium longum DS0950 Culture-Supernatants Ameliorate Obesity-Related Characteristics in Mice with High-Fat Diet-Induced Obesity.J Microbiol Biotechnol. 2023 Jan 28;33(1):96-105. doi: 10.4014/jmb.2210.10046. Epub 2022 Nov 22. J Microbiol Biotechnol. 2023. PMID: 36457182 Free PMC article.
-
Crotamine induces browning of adipose tissue and increases energy expenditure in mice.Sci Rep. 2018 Mar 22;8(1):5057. doi: 10.1038/s41598-018-22988-1. Sci Rep. 2018. PMID: 29567992 Free PMC article.
-
A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate.Cell Metab. 2019 Jul 2;30(1):174-189.e5. doi: 10.1016/j.cmet.2019.05.005. Epub 2019 May 30. Cell Metab. 2019. PMID: 31155495 Free PMC article.
-
Maternal glycemia in pregnancy is longitudinally associated with blood DNAm variation at the FSD1L gene from birth to 5 years of age.Clin Epigenetics. 2023 Jun 29;15(1):107. doi: 10.1186/s13148-023-01524-7. Clin Epigenetics. 2023. PMID: 37386647 Free PMC article.
-
Prdm16 is required for the maintenance of neural stem cells in the postnatal forebrain and their differentiation into ependymal cells.Genes Dev. 2017 Jun 1;31(11):1134-1146. doi: 10.1101/gad.291773.116. Epub 2017 Jul 11. Genes Dev. 2017. PMID: 28698301 Free PMC article.
References
-
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007; 6:38-54; PMID:17618855; http://dx.doi.org/10.1016/j.cmet.2007.06.001 - DOI - PMC - PubMed
-
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277-359; PMID:14715917; http://dx.doi.org/10.1152/physrev.00015.2003 - DOI - PubMed
-
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. . Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/10.1016/j.cell.2012.05.016 - DOI - PMC - PubMed
-
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. . PRDM16 controls a brown fatskeletal muscle switch. Nature 2008; 454:961-7; PMID:18719582; http://dx.doi.org/10.1038/nature07182 - DOI - PMC - PubMed
-
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. . A smooth muscle-like origin for beige adipocytes. Cell Metab 2014; 19:810-20; PMID:24709624; http://dx.doi.org/10.1016/j.cmet.2014.03.025 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
