The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm
- PMID: 27226326
- PMCID: PMC4958327
- DOI: 10.1242/dev.136499
The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm
Abstract
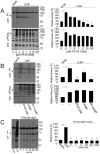
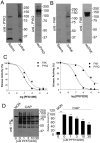
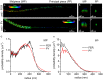
Sperm capacitation is required for fertilization. At the molecular level, this process is associated with fast activation of protein kinase A. Downstream of this event, capacitating conditions lead to an increase in tyrosine phosphorylation. The identity of the tyrosine kinase(s) mediating this process has not been conclusively demonstrated. Recent experiments using stallion and human sperm have suggested a role for PYK2 based on the use of small molecule inhibitors directed against this kinase. However, crucially, loss-of-function experiments have not been reported. Here, we used both pharmacological inhibitors and genetically modified mice models to investigate the identity of the tyrosine kinase(s) mediating the increase in tyrosine phosphorylation in mouse sperm. Similar to stallion and human, PF431396 blocks the capacitation-associated increase in tyrosine phosphorylation. Yet, sperm from Pyk2(-/-) mice displayed a normal increase in tyrosine phosphorylation, implying that PYK2 is not responsible for this phosphorylation process. Here, we show that PF431396 can also inhibit FER, a tyrosine kinase known to be present in sperm. Sperm from mice targeted with a kinase-inactivating mutation in Fer failed to undergo capacitation-associated increases in tyrosine phosphorylation. Although these mice are fertile, their sperm displayed a reduced ability to fertilize metaphase II-arrested eggs in vitro.
Keywords: Capacitation; FER; Tyrosine phosphorylation.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



Similar articles
-
Semi-automatized segmentation method using image-based flow cytometry to study sperm physiology: the case of capacitation-induced tyrosine phosphorylation.Mol Hum Reprod. 2018 Feb 1;24(2):64-73. doi: 10.1093/molehr/gax062. Mol Hum Reprod. 2018. PMID: 29186618 Free PMC article.
-
Capacitation is associated with tyrosine phosphorylation and tyrosine kinase-like activity of pig sperm proteins.Biol Reprod. 2001 Sep;65(3):784-92. doi: 10.1095/biolreprod65.3.784. Biol Reprod. 2001. PMID: 11514342
-
Evidence for the involvement of proline-rich tyrosine kinase 2 in tyrosine phosphorylation downstream of protein kinase A activation during human sperm capacitation.Mol Hum Reprod. 2014 Nov;20(11):1054-66. doi: 10.1093/molehr/gau073. Epub 2014 Sep 1. Mol Hum Reprod. 2014. PMID: 25180269 Free PMC article.
-
Kinases, phosphatases and proteases during sperm capacitation.Cell Tissue Res. 2012 Sep;349(3):765-82. doi: 10.1007/s00441-012-1370-3. Epub 2012 Mar 20. Cell Tissue Res. 2012. PMID: 22427115 Review.
-
Use of phosphoproteomics to study tyrosine kinase activity in capacitating boar sperm. Kinase activity and capacitation.Theriogenology. 2005 Jan 15;63(2):599-614. doi: 10.1016/j.theriogenology.2004.09.034. Theriogenology. 2005. PMID: 15626419 Review.
Cited by
-
The protein phosphatase isoform PP1γ1 substitutes for PP1γ2 to support spermatogenesis but not normal sperm function and fertility†.Biol Reprod. 2019 Mar 1;100(3):721-736. doi: 10.1093/biolre/ioy225. Biol Reprod. 2019. PMID: 30379985 Free PMC article.
-
C2CD6 regulates targeting and organization of the CatSper calcium channel complex in sperm flagella.Development. 2022 Jan 15;149(2):dev199988. doi: 10.1242/dev.199988. Epub 2022 Jan 14. Development. 2022. PMID: 34919125 Free PMC article.
-
The secrets of success.Elife. 2020 Dec 2;9:e64379. doi: 10.7554/eLife.64379. Elife. 2020. PMID: 33263540 Free PMC article.
-
Exploring the ovine sperm transcriptome by RNAseq techniques. I Effect of seasonal conditions on transcripts abundance.PLoS One. 2022 Mar 14;17(3):e0264978. doi: 10.1371/journal.pone.0264978. eCollection 2022. PLoS One. 2022. PMID: 35286314 Free PMC article.
-
Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains.Cells. 2019 Jun 27;8(7):648. doi: 10.3390/cells8070648. Cells. 2019. PMID: 31252584 Free PMC article.
References
-
- Austin C. R. (1951). Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. Ser. B Biol. Sci. 4, 581-596. - PubMed
-
- Baldi E., Casano R., Falsetti C., Krausz C., Maggi M. and Forti G. (1991). Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl. 12, 323-330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

