Flow-dependent myosin recruitment during Drosophila cellularization requires zygotic dunk activity
- PMID: 27226317
- PMCID: PMC4958320
- DOI: 10.1242/dev.131334
Flow-dependent myosin recruitment during Drosophila cellularization requires zygotic dunk activity
Abstract
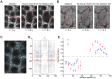
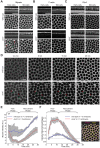
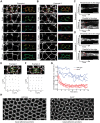
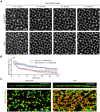
Actomyosin contractility underlies force generation in morphogenesis ranging from cytokinesis to epithelial extension or invagination. In Drosophila, the cleavage of the syncytial blastoderm is initiated by an actomyosin network at the base of membrane furrows that invaginate from the surface of the embryo. It remains unclear how this network forms and how it affects tissue mechanics. Here, we show that during Drosophila cleavage, myosin recruitment to the cleavage furrows proceeds in temporally distinct phases of tension-driven cortical flow and direct recruitment, regulated by different zygotic genes. We identify the gene dunk, which we show is transiently transcribed when cellularization starts and functions to maintain cortical myosin during the flow phase. The subsequent direct myosin recruitment, however, is Dunk-independent but requires Slam. The Slam-dependent direct recruitment of myosin is sufficient to drive cleavage in the dunk mutant, and the subsequent development of the mutant is normal. In the dunk mutant, cortical myosin loss triggers misdirected flow and disrupts the hexagonal packing of the ingressing furrows. Computer simulation coupled with laser ablation suggests that Dunk-dependent maintenance of cortical myosin enables mechanical tension build-up, thereby providing a mechanism to guide myosin flow and define the hexagonal symmetry of the furrows.
Keywords: Actomyosin network; Cellularization; Cortical myosin recruitment; Cytokinesis; Dunk.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




Similar articles
-
Early zygotic gene product Dunk interacts with anillin to regulate Myosin II during Drosophila cleavage.Mol Biol Cell. 2023 Sep 1;34(10):ar102. doi: 10.1091/mbc.E22-02-0046. Epub 2023 Jul 26. Mol Biol Cell. 2023. PMID: 37494082 Free PMC article.
-
Spatiotemporal recruitment of RhoGTPase protein GRAF inhibits actomyosin ring constriction in Drosophila cellularization.Elife. 2021 Apr 9;10:e63535. doi: 10.7554/eLife.63535. Elife. 2021. PMID: 33835025 Free PMC article.
-
Cross-linker-mediated regulation of actin network organization controls tissue morphogenesis.J Cell Biol. 2019 Aug 5;218(8):2743-2761. doi: 10.1083/jcb.201811127. Epub 2019 Jun 28. J Cell Biol. 2019. PMID: 31253650 Free PMC article.
-
Membrane-actin interactions in morphogenesis: Lessons learned from Drosophila cellularization.Semin Cell Dev Biol. 2023 Jan 15;133:107-122. doi: 10.1016/j.semcdb.2022.03.028. Epub 2022 Apr 5. Semin Cell Dev Biol. 2023. PMID: 35396167 Free PMC article. Review.
-
Reshaping the Syncytial Drosophila Embryo with Cortical Actin Networks: Four Main Steps of Early Development.Results Probl Cell Differ. 2024;71:67-90. doi: 10.1007/978-3-031-37936-9_4. Results Probl Cell Differ. 2024. PMID: 37996673 Review.
Cited by
-
Disease-related non-muscle myosin IIA D1424N rod domain mutation, but not R702C motor domain mutation, disrupts mouse ocular lens fiber cell alignment and hexagonal packing.Cytoskeleton (Hoboken). 2024 Dec;81(12):789-805. doi: 10.1002/cm.21853. Epub 2024 Mar 22. Cytoskeleton (Hoboken). 2024. PMID: 38516850
-
Mitochondrial morphology and activity regulate furrow ingression and contractile ring dynamics in Drosophila cellularization.Mol Biol Cell. 2020 Oct 1;31(21):2331-2347. doi: 10.1091/mbc.E20-03-0177. Epub 2020 Aug 5. Mol Biol Cell. 2020. PMID: 32755438 Free PMC article.
-
Curvature gradient drives polarized tissue flow in the Drosophila embryo.Proc Natl Acad Sci U S A. 2023 Feb 7;120(6):e2214205120. doi: 10.1073/pnas.2214205120. Epub 2023 Feb 1. Proc Natl Acad Sci U S A. 2023. PMID: 36724258 Free PMC article.
-
-Back-to-back mechanisms drive actomyosin ring closure during Drosophila embryo cleavage.J Cell Biol. 2016 Nov 7;215(3):335-344. doi: 10.1083/jcb.201608025. Epub 2016 Oct 31. J Cell Biol. 2016. PMID: 27799369 Free PMC article.
-
Toll-Dorsal signaling regulates the spatiotemporal dynamics of yolk granule tubulation during Drosophila cleavage.Dev Biol. 2022 Jan;481:64-74. doi: 10.1016/j.ydbio.2021.09.009. Epub 2021 Oct 7. Dev Biol. 2022. PMID: 34627795 Free PMC article.
References
-
- Afshar K., Stuart B. and Wasserman S. A. (2000). Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development 127, 1887-1897. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

